Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation - PubMed (original) (raw)
Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation
J C Yoon et al. Mol Cell Biol. 2000 Jul.
Abstract
The nuclear receptor peroxisome proliferator-activated receptor gamma regulates adipose differentiation and systemic insulin signaling via ligand-dependent transcriptional activation of target genes. However, the identities of the biologically relevant target genes are largely unknown. Here we describe the isolation and characterization of a novel target gene induced by PPARgamma ligands, termed PGAR (for PPARgamma angiopoietin related), which encodes a novel member of the angiopoietin family of secreted proteins. The transcriptional induction of PGAR follows a rapid time course typical of immediate-early genes and occurs in the absence of protein synthesis. The expression of PGAR is predominantly localized to adipose tissues and placenta and is consistently elevated in genetic models of obesity. Hormone-dependent adipocyte differentiation coincides with a dramatic early induction of the PGAR transcript. Alterations in nutrition and leptin administration are found to modulate the PGAR expression in vivo. Taken together, these data suggest a possible role for PGAR in the regulation of systemic lipid metabolism or glucose homeostasis.
Figures
FIG. 1
Sequence analysis of PGAR. (A) Deduced amino acid sequences of mouse PGAR (mPGAR) and human PGAR (hPGAR). The arrows indicate the limits of the coiled-coil and fibrinogen-like domains. (B) Schematic diagram of the predicted PGAR protein structure. (C) Alignment of the fibrinogen-like domains (FLD) of PGAR and angiopoietin-2 (Ang2). Conserved cysteines are indicated by solid circles. SS, signal sequence. (D) Genomic structure of PGAR. Exons are denoted by black boxes, and introns are denoted by a solid line.
FIG. 2
Detection of PGAR in secreted form. Shown is a Western blot of conditioned media and cell lysates of COS7 cells transiently transfected with HA-tagged PGAR construct (+) or an empty vector (−). IVT, 10 μl of in vitro transcription and translation products of pcDNA and pcDNA-PGAR-HA, respectively, was used with the TNT in vitro translation system (Promega); CM, 50 μl of 10-fold-concentrated supernatant collected from pcDNA and pcDNA-PGAR-HA transfected cells was used; lysate, 10 μl of cell lysates from the transfected cells was used. See Materials and Methods for additional details.
FIG. 3
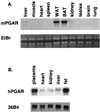
Tissue expression of PGAR. (A) RNA blot analysis of mouse PGAR (mPGAR) in adult mouse tissues. Ten micrograms of total RNA from tissues of 9-week-old male C57BL KS/J mice was fractionated on an agarose gel and transferred to nylon membranes. WAT, white adipose tissue; BAT, brown adipose tissue; EtBr, ethidium bromide staining. (B) Expression of human PGAR (hPGAR) RNA. Each lane contains 10 μg of total RNA prepared from surgical specimens (Brigham and Women's Hospital, Boston, Mass.).
FIG. 4
In situ analysis of PGAR in mouse. Shown are parasagittal sections (8 μm thick) of E18.5 mouse embryo hybridized to antisense (B) or sense (C) PGAR RNA probe. Arrows point to subscapular brown fat (bf) and liver (lv). Also shown is a bright-field image of a hematoxylin-and-eosin-stained section (A).
FIG. 5
Regulation of PGAR mRNA in PPARγ-expressing cells. NIH 3T3 fibroblasts were stably infected with retroviruses carrying pBabe-PPARγ and were cultured in 10% fetal bovine serum–DMEM. Cells were pretreated with cycloheximide (CHX) (5 μg/ml) (lanes 1 to 3 and 7 to 9) or vehicle (lanes 4 to 6 and 10 to 12) for 15 min and subsequently incubated further with pioglitazone (pio) (10 μg/ml) (lanes 1 to 6) or vehicle (lanes 7 to 12). Total RNA was isolated 0, 2, or 4 h after initiation of pioglitazone treatment and analyzed by Northern blotting (20 μg/lane). EtBr, ethidium bromide staining.
FIG. 6
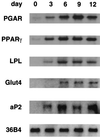
Induction of PGAR expression during adipocyte differentiation. 3T3-L1 preadipocytes were cultured to confluence and induced to differentiate using established protocols, i.e., 3 days of dexamethasone-isobutylmethylxanthine-insulin treatment followed by insulin treatment. Northern analysis was performed on the total RNA isolated 3, 6, 9, and 12 days after induction (10 μg/lane).
FIG. 7
Regulation of PGAR in mouse models of obesity and by nutritional changes and leptin administration. (A) Northern analysis of PGAR (10 μg RNA/lane) in white (WAT) and brown (BAT) fat tissues from 9-week-old obese (ob/ob) and diabetic (db/db) mice and their congenic controls. (B) PGAR expression in white adipose tissue of mice subjected to dietary restrictions. Eleven-week-old C57BL KS/J mice were divided into three experimental groups and were either allowed free access to food (lane 1) or subjected to 12 h of fast (lane 2) or 7.5 h of fast followed by refeeding (lane 3). The animals were sacrificed at the end of 12 h, and 10 μg of total RNA extracted from pooled white fat (n = 3 per group) was loaded on each lane. (C) PGAR expression in leptin-treated versus diet-restricted animals. Eight-week-old female C57BL/6J mice were divided into three groups (n = 5). The first two groups received intraperitoneal injections twice daily with either PBS or leptin. The third group, the pair-fed group, was diet-restricted to match the amount of food intake by the leptin-injected group and also received injections with PBS. The animals were sacrificed after 1 or 5 days, and 10 μg of total RNA from pooled white fat was analyzed by Northern blotting. See Materials and Methods for additional details.
References
- Ausubel F, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1991.
- Barak Y, Nelson M C, Ong E S, Jones Y Z, Ruiz-Lozano P, Chien K R, Koder A, Evans R M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. - PubMed
- Barroso I, Gurnell M, Crowley V E F, Agnosti M, Schwabe J W, Soos M A, Maslen G L, Williams T D M, Lewis H, Schafer A J, Chatterjee V K K, O'Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus, and hypertension. Nature. 1999;402:880–883. - PubMed
- Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-delta 12, 14-prostaglandin J2. J Biol Chem. 1999;274:17042–17048. - PubMed
- Busfield S J, Michnick D A, Chickering T W, Revett T L, Ma J, Woolf E A, Comrack C A, Dussault B J, Woolf J, Goodearl A D J, Gearing D P. Characterization of a neuregulin-related gene, Don-1, that is highly expressed in restricted regions of the cerebellum and hippocampus. Mol Cell Biol. 1997;17:4007–4014. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases