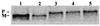Green fluorescent protein functions as a reporter for protein localization in Escherichia coli - PubMed (original) (raw)
Green fluorescent protein functions as a reporter for protein localization in Escherichia coli
B J Feilmeier et al. J Bacteriol. 2000 Jul.
Abstract
The use of green fluorescent protein (GFP) as a reporter for protein localization in Escherichia coli was explored by creating gene fusions between malE (encoding maltose-binding protein [MBP]) and a variant of gfp optimized for fluorescence in bacteria (GFPuv). These constructs encode hybrid proteins composed of GFP fused to the carboxy-terminal end of MBP. Fluorescence was not detected when the hybrid protein was synthesized with the MBP signal sequence. In contrast, when the MBP signal sequence was deleted, fluorescence was observed. Cell fractionation studies showed that the fluorescent MBP-GFP hybrid protein was localized in the cytoplasm, whereas the nonfluorescent version was localized to the periplasmic space. Smaller MBP-GFP hybrid proteins, however, exhibited abnormal fractionation. Expression of the gene fusions in different sec mutants, as well as signal sequence processing assays, confirmed that the periplasmically localized hybrid proteins were exported by the sec-dependent pathway. The distinction between fluorescent and nonfluorescent colonies was exploited as a scorable phenotype to isolate malE signal sequence mutations. While expression of hybrid proteins comprised of full-length MBP did not result in overproduction lethality characteristic of some exported beta-galactosidase hybrid proteins, synthesis of shorter, exported hybrid proteins was toxic to the cells. Purification of MBP-GFP hybrid protein from the different cellular compartments indicated that GFP is improperly folded when localized outside of the cytoplasm. These results suggest that GFP could serve as a useful reporter for genetic analysis of bacterial protein export and of protein folding.
Figures
FIG. 1
Constructs encoding MBP-GFP hybrid proteins. (A) Constructs carried by pMGP2 (encoding MBP[SS418]) and pMGC2 (encoding MBP[ΔSS418]). (B) Constructs carried by pMGP22 (encoding MBP[SS128]) and pMGC22 (encoding MBP[ΔSS128]). Features: lacI (diagonal stripes), LacI repressor; malE_′ (narrow vertical stripes), MBP; ′_gfp, GFPuv (wide vertical stripes); POlac, lac promoter and operator; SS (horizontal stripes), signal sequence encoding region; and the linker region between malE and gfp (solid black line). The location of the region of gfp encoding the chromophore is indicated by an asterisk. Relevant restriction sites: E, _Eco_RV; B, _Bam_HI; Bg, _Bgl_II; H, _Hin_dIII. Δ, region of malE deleted in the constructs. The designation of the hybrid proteins encoded by each construct is shown on the right. The number within the square brackets indicates the number of amino acids of the mature portion of MBP fused to GFP.
FIG. 2
Fluorescence of transformants expressing MBP-GFP hybrid proteins. MC4100 transformed with the following: 1, pMGP2; 2, pMGC2; 8, MM52 [secA(Ts)] transformed with pMGP2; 3, pMGC2; 7, CK2163 (secB) transformed with pMGP2; 4, pMGC2; 6, IQ85 [secY(Ts)] transformed with pMGP2; and 5, pMGC2.
FIG. 3
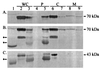
Immunoblot analysis of MBP-GFP hybrid proteins. Cells transformed with plasmids expressing either MBP-GFP hybrid proteins (A and B) or MBP (C) were fractionated as described in Materials and Methods and analyzed by Western blot analysis. Immunoblots were decorated with anti-GFP antibody (A) or anti-MBP antibody (B and C). For panels A and B, lanes represent transformants of the following: lane 1, pJF2 (expressing wild-type MBP); lanes 2, 4, 6, and 8, pMGC2 (expressing MBP[Δ418]-GFP); lanes 3, 5, 7, and 9, pMGP2 (expressing MBP[SS418]-GFP). (C) MBP expressed from: lane 1, pJF2; lanes 2, 4, 6, and 8, pMalc2; lanes 3, 5, 7, and 9, pMalp2. Lane designations: WC, whole cell; P, periplasmic fraction; C, cytoplasmic fraction; M, membrane fraction. The location of the full-length MBP-GFP protein is noted with arrows next to the nearest corresponding molecular weight marker; the asterisk indicates breakdown products detected with anti-MBP antibody.
FIG. 4
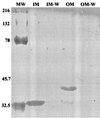
Cell fractionation of shortened MBP-GFP hybrid protein. Cells expressing MBP[SS128]-GFP were fractionated into inner and outer membranes as described in Materials and Methods. The immunoblot shown was decorated with anti-GFP antibody. MBP[SS128]-GFP migrated at a predicted molecular mass of 40 kDa and fractionated with the outer membrane. An apparent smaller-molecular-weight derivative of this protein was also found in the inner membrane fraction. Molecular weight (MW) standards are as shown. IM, inner membrane fraction; IM-W, KCl wash of inner membrane fraction; OM, outer membrane fraction; OM-W, KCl wash of outer membrane fraction. Although not shown, OmpA and ATPase were used to monitor fractionation of the outer membrane and inner membranes, respectively.
FIG. 5
Signal sequence mutations of MBP. The wild-type malE signal sequence encoding region is shown below the amino acid sequence of the amino-terminal region of the precursor MBP protein along with the sequence of three signal sequence mutations.
FIG. 6
Signal sequence processing of MBP-GFP hybrid proteins. Shown are pulse-labeled MBP-GFP proteins immune precipitated with anti-GFP antibody. Transformants labeled: lane 1, pMGC2; lane 2, pMGP2; lane 3, pMGP2 (mutant M1); lane 4, pMGP2 (mutant M2.1); lane 5, pMGP2 (mutant M2.3). P, precursor (signal sequence unprocessed) form; M, mature form.
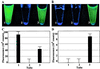
FIG. 7
Characterization of purified MBP-GFP hybrid protein. Hybrid proteins were purified from the periplasmic space or the cytoplasm and subjected to acid-base treatment, as described in Materials and Methods. (A) MBP[ΔSS418]-GFP isolated from the cytoplasm. Tubes: 1, untreated; 2, acid treated; 3, acid-base treated. (B) MBP[SS418]-GFP isolated from the periplasmic space. Tubes: 1, untreated; 2, acid treated; 3, acid-base treated. (C) Relative fluorescence of tubes shown in panel A. (D) Relative fluorescence of tubes shown in panel B.
Similar articles
- Export of the periplasmic maltose-binding protein of Escherichia coli.
Bassford PJ Jr. Bassford PJ Jr. J Bioenerg Biomembr. 1990 Jun;22(3):401-39. doi: 10.1007/BF00763175. J Bioenerg Biomembr. 1990. PMID: 2202725 Review. - SecB-independent export of Escherichia coli ribose-binding protein (RBP): some comparisons with export of maltose-binding protein (MBP) and studies with RBP-MBP hybrid proteins.
Collier DN, Strobel SM, Bassford PJ Jr. Collier DN, et al. J Bacteriol. 1990 Dec;172(12):6875-84. doi: 10.1128/jb.172.12.6875-6884.1990. J Bacteriol. 1990. PMID: 2254262 Free PMC article. - Alterations in the hydrophilic segment of the maltose-binding protein (MBP) signal peptide that affect either export or translation of MBP.
Puziss JW, Harvey RJ, Bassford PJ Jr. Puziss JW, et al. J Bacteriol. 1992 Oct;174(20):6488-97. doi: 10.1128/jb.174.20.6488-6497.1992. J Bacteriol. 1992. PMID: 1400201 Free PMC article. - Maltose transport system of Escherichia coli: an ABC-type transporter.
Nikaido H. Nikaido H. FEBS Lett. 1994 Jun 6;346(1):55-8. doi: 10.1016/0014-5793(94)00315-7. FEBS Lett. 1994. PMID: 8206159 Review.
Cited by
- Bacteria-Based Living Probes: Preparation and the Applications in Bioimaging and Diagnosis.
Jiang H, Cao Z, Liu Y, Liu R, Zhou Y, Liu J. Jiang H, et al. Adv Sci (Weinh). 2024 Jan;11(4):e2306480. doi: 10.1002/advs.202306480. Epub 2023 Nov 30. Adv Sci (Weinh). 2024. PMID: 38032119 Free PMC article. Review. - AAV Vectors for FRET-Based Analysis of Protein-Protein Interactions in Photoreceptor Outer Segments.
Becirovic E, Böhm S, Nguyen ON, Riedmayr LM, Hammelmann V, Schön C, Butz ES, Wahl-Schott C, Biel M, Michalakis S. Becirovic E, et al. Front Neurosci. 2016 Jul 28;10:356. doi: 10.3389/fnins.2016.00356. eCollection 2016. Front Neurosci. 2016. PMID: 27516733 Free PMC article. - Application of split-green fluorescent protein for topology mapping membrane proteins in Escherichia coli.
Toddo S, Söderström B, Palombo I, von Heijne G, Nørholm MH, Daley DO. Toddo S, et al. Protein Sci. 2012 Oct;21(10):1571-6. doi: 10.1002/pro.2131. Epub 2012 Aug 21. Protein Sci. 2012. PMID: 22825803 Free PMC article. - The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides.
Suaste-Olmos F, Domenzain C, Mireles-Rodríguez JC, Poggio S, Osorio A, Dreyfus G, Camarena L. Suaste-Olmos F, et al. J Bacteriol. 2010 Dec;192(23):6230-9. doi: 10.1128/JB.00655-10. Epub 2010 Oct 1. J Bacteriol. 2010. PMID: 20889747 Free PMC article. - Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles.
Kesty NC, Kuehn MJ. Kesty NC, et al. J Biol Chem. 2004 Jan 16;279(3):2069-76. doi: 10.1074/jbc.M307628200. Epub 2003 Oct 24. J Biol Chem. 2004. PMID: 14578354 Free PMC article.
References
- Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. - PubMed
- Bassford P, Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979;277:538–541. - PubMed
- Bedouelle H, Bassford P J, Jr, Fowler A V, Zabin I, Beckwith J, Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980;285:78–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous