Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation - PubMed (original) (raw)
Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation
F Wei et al. J Cell Biol. 2000.
Abstract
Hippocampal neurons fire spikes when an animal is at a particular location or performs certain behaviors in a particular place, providing a cellular basis for hippocampal involvement in spatial learning and memory. In a natural environment, spatial memory is often associated with potentially dangerous sensory experiences such as noxious or painful stimuli. The central sites for such pain-associated memory or plasticity have not been identified. Here we present evidence that excitatory glutamatergic synapses within the CA1 region of the hippocampus may play a role in storing pain-related information. Peripheral noxious stimulation induced excitatory postsynaptic potentials (EPSPs) in CA1 pyramidal cells in anesthetized animals. Tissue/nerve injury caused a rapid increase in the level of the immediate-early gene product Egr1 (also called NGFI-A, Krox24, or zif/268) in hippocampal CA1 neurons. In parallel, synaptic potentiation induced by a single tetanic stimulation (100 Hz for 1 s) was enhanced after the injury. This enhancement of synaptic potentiation was absent in mice lacking Egr1. Our data suggest that Egr1 may act as an important regulator of pain-related synaptic plasticity within the hippocampus.
Figures
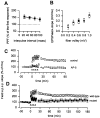
Figure 1
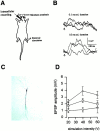
Peripheral noxious stimuli induced EPSPs from CA1 pyramidal neurons. (A) Diagram of an in vivo intracellular recording in an anesthetized rat. (C) An example of intracellularly stained CA1 pyramidal neurons (C). Representative traces (B) show the evoked responses of a CA1 neuron to stimuli of different durations. Each is the average of four traces. (Arrow) The stimulus artifact. (D) Plot of EPSP amplitude versus intensity of peripheral stimulation with different stimulus durations (triangles: 1.0 ms; squares: 0.5 ms; circles: 0.1 ms). Each point is the mean ± SEM.
Figure 2
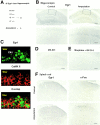
Amputation of a mouse distal tail segment increased hippocampal Egr1. Egr1 was isolated by immunoprecipitation from hippocampus and detected by Western blot in control mice (indicated by −) and mice 1 h after amputation (+). Increases in hippocampal Egr1 immunoreactivity at 45 min after amputation are compared with the hippocampus of normal mice (the bottom set of photos are high-magnification details of the indicated areas). Confocal images of double-labeled CA1 pyramidal neurons in the hippocampus of amputated mice for FITC-labeled Egr1 (green, top), Cy-3 labeled CaMKII (red, middle), and merged image (bottom) showing a strong nuclear Egr1 signal expression in many pyramidal neurons visualized by CaMKII immunofluorescence. (D) Pretreatment with MK-801 (1 mg/kg) almost completely blocked Egr1 activation. (E) Intraperitoneal morphine (10 mg/kg) and subcutaneous QX-314 (5%, 10 μl) significantly decreased amputation-induced Egr1 activation. (F) Amputation increased c-Fos but not Egr1 immunoreactivity in the spinal dorsal horn. Bars: (B, top and E) 400 μm; (B, bottom) 150 μm; (F) 100 μm.
Figure 3
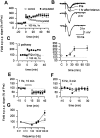
Amputation affected hippocampal LTP but not LTD. A single tetanic stimulation (100 Hz, 1 s) produced short-term potentiation in normal mice (n = 6, open squares). But 45 min after amputation, tetanic stimulation caused enhanced synaptic potentiation lasting for at least 60 min (n = 6, filled squares). An example illustrates that synaptic potentiation in slices prepared from amputated mice persisted for at least 3 h. Synaptic potentiation is input specific. As in A, a single tetanic stimulation induced enhanced potentiation (n = 4, filled squares) but synaptic responses at the second, independent pathway remained unaffected (open squares). Summarized time course curve of the effect of amputation on synaptic potentiation induced by one train tetanic stimulation (filled squares; open circle indicates sham-animals). Keeping mice under general anesthesia during the 45 min between amputation and decapitation prevented synaptic potentiation caused by amputation (filled triangles). LTD was not affected by amputation (control, n = 7, open squares; amputated, n = 5, filled squares). Synaptic responses to 5 Hz stimulation (for 3 min) also revealed no difference (control: n = 5, 80.0 ± 14.5%, open squares; amputated: n = 4, 94.2 ± 20.5%, filled squares). Summary of frequency-dependent responses.
Figure 4
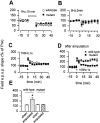
Hippocampal LTP and LTD in mice lacking Egr1. LTD was normal in mutant mice (wild-type, n = 8, open squares; mutant, n = 6, filled squares). Synaptic responses to 5 Hz stimulation was also normal (wild-type, n = 4, open squares; mutant, n = 5, filled squares). Synaptic potentiation induced by a single tetanic stimulation was similar (wild-type, n = 8, open squares; mutant, n = 5, filled squares). Amputation caused no synaptic enhancement of LTP in mutant mice (wild-type, n = 5, open squares; mutant, n = 6, filled squares). Summarized data of different treatments on the enhancement of LTP caused by amputation.
Figure 5
Egr1 contributes to NMDA receptor-dependent late-phase LTP. (A) Wild-type (n = 6, open squares) and mutant slices (n = 8, filled squares) showed no significant difference in paired-pulse facilitation of the field EPSP at different interpulse intervals. (B) Wild-type (n = 9, open squares) and mutant slices (n = 6, filled squares) showed no significant difference in NMDA receptor-mediated EPSPs. (C) The induction of late-phase LTP in wild-type mice was completely blocked by 100 μM AP-5 in bath solution (n = 4). (D) Late-phase LTP was significantly decreased in mutant mice (wild-type, n = 9, open squares; mutant, n = 5, filled squares).
Similar articles
- Subfield-specific immediate early gene expression associated with hippocampal long-term potentiation in vivo.
French PJ, O'Connor V, Jones MW, Davis S, Errington ML, Voss K, Truchet B, Wotjak C, Stean T, Doyère V, Maroun M, Laroche S, Bliss TV. French PJ, et al. Eur J Neurosci. 2001 Mar;13(5):968-76. doi: 10.1046/j.0953-816x.2001.01467.x. Eur J Neurosci. 2001. PMID: 11264669 - Long-term potentiation and long-term depression in hippocampal CA1 neurons of mice lacking the IP(3) type 1 receptor.
Nagase T, Ito KI, Kato K, Kaneko K, Kohda K, Matsumoto M, Hoshino A, Inoue T, Fujii S, Kato H, Mikoshiba K. Nagase T, et al. Neuroscience. 2003;117(4):821-30. doi: 10.1016/s0306-4522(02)00803-5. Neuroscience. 2003. PMID: 12654335 - Muscarinic Receptors, from Synaptic Plasticity to its Role in Network Activity.
Fernández de Sevilla D, Núñez A, Buño W. Fernández de Sevilla D, et al. Neuroscience. 2021 Feb 21;456:60-70. doi: 10.1016/j.neuroscience.2020.04.005. Epub 2020 Apr 8. Neuroscience. 2021. PMID: 32278062 Review. - Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.
MacDonald JF, Jackson MF, Beazely MA. MacDonald JF, et al. Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
- DREAM (downstream regulatory element antagonist modulator) contributes to synaptic depression and contextual fear memory.
Wu LJ, Mellström B, Wang H, Ren M, Domingo S, Kim SS, Li XY, Chen T, Naranjo JR, Zhuo M. Wu LJ, et al. Mol Brain. 2010 Jan 21;3:3. doi: 10.1186/1756-6606-3-3. Mol Brain. 2010. PMID: 20205763 Free PMC article. - Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation.
Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Wu LJ, et al. J Neurosci. 2005 Nov 30;25(48):11107-16. doi: 10.1523/JNEUROSCI.1678-05.2005. J Neurosci. 2005. PMID: 16319310 Free PMC article. - 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats.
Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC. Wu YW, et al. J Neurosci. 2010 Jun 30;30(26):8710-9. doi: 10.1523/JNEUROSCI.6323-09.2010. J Neurosci. 2010. PMID: 20592193 Free PMC article. - Amygdaloid zif268 participated in the D-cycloserine facilitation effect on the extinction of conditioned fear.
Wu IT, Tang TH, Ko MC, Chiu CY, Lu KT. Wu IT, et al. Psychopharmacology (Berl). 2015 Oct;232(20):3809-19. doi: 10.1007/s00213-015-4042-7. Epub 2015 Aug 19. Psychopharmacology (Berl). 2015. PMID: 26282370 - EGR1 recruits TET1 to shape the brain methylome during development and upon neuronal activity.
Sun Z, Xu X, He J, Murray A, Sun MA, Wei X, Wang X, McCoig E, Xie E, Jiang X, Li L, Zhu J, Chen J, Morozov A, Pickrell AM, Theus MH, Xie H. Sun Z, et al. Nat Commun. 2019 Aug 29;10(1):3892. doi: 10.1038/s41467-019-11905-3. Nat Commun. 2019. PMID: 31467272 Free PMC article.
References
- Aloisi A.M., Zimmermann M., Herdegen T. Sex-dependent effects of formalin and restraint on c-Fos expression in the septum and hippocampus of the rat. Neuroscience. 1997;81:951–958. - PubMed
- Bear M.F., Malenka R.C. Synaptic plasticityLTP and LTD. Curr. Opin. Neurobiol. 1994;4:389–399. - PubMed
- Berger T.W., Alger B., Thompson R.F. Neuronal substrate of classical conditioning in the hippocampus. Science. 1976;192:483–485. - PubMed
- Berger T.W., Laham R.I., Thompson R.F. Hippocampal unit-behavior correlations during classical conditioning. Brain Res. 1980;193:229–248. - PubMed
- Berger T.W., Rinaldi P.C., Weisz D.J., Thompson R.F. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J. Neurophysiol. 1983;50:1197–1219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous