Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex - PubMed (original) (raw)
Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex
A D Rudner et al. J Cell Biol. 2000.
Abstract
Budding yeast initiates anaphase by activating the Cdc20-dependent anaphase-promoting complex (APC). The mitotic activity of Cdc28 (Cdk1) is required to activate this form of the APC, and mutants that are impaired in mitotic Cdc28 function have difficulty leaving mitosis. This defect can be explained by a defect in APC phosphorylation, which depends on mitotic Cdc28 activity in vivo and can be catalyzed by purified Cdc28 in vitro. Mutating putative Cdc28 phosphorylation sites in three components of the APC, Cdc16, Cdc23, and Cdc27, makes the APC resistant to phosphorylation both in vivo and in vitro. The nonphosphorylatable APC has normal activity in G1, but its mitotic, Cdc20-dependent activity is compromised. These results show that Cdc28 activates the APC in budding yeast to trigger anaphase. Previous reports have shown that the budding yeast Cdc5 homologue, Plk, can also phosphorylate and activate the APC in vitro. We show that, like cdc28 mutants, cdc5 mutants affect APC phosphorylation in vivo. However, although Cdc5 can phosphorylate Cdc16 and Cdc27 in vitro, this in vitro phosphorylation does not occur on in vivo sites of phosphorylation.
Figures
Figure 1
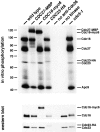
The APC is phosphorylated in vitro by Cdc28. Wild-type (ADR376), CDC27-MBP (ADR1705), CDC16-myc6 (K6180), CDC23-HA (SLJ378), and cdc5-1 (JC145) were grown overnight in YEP + 2% glucose at 23°C to log phase, arrested in G1 by alpha factor (1 μg/ml) for 3 h, harvested, lysed, and the APC immunoprecipitated with anti-Cdc26 antibody. The immunoprecipitates were treated with purified Cdc28-His6, Clb2-MBP, Cks1, and γ[32P]ATP, were washed to remove phosphorylated Clb2-MBP, and were then run on a polyacrylamide gel that was subjected to autoradiography (top) or Western blotting (bottom). cdc5-1 cells were shifted to 37°C for an additional 1 h of alpha factor treatment. As controls, cell lysate was mock precipitated in the absence of anti-Cdc26 antibody (no anti-Cdc26) or was precipitated in the presence of anti-Cdc26 antibody, but no Cdc28, Clb2, or Cks1 was added to kinase reaction (no kinase). The Western blot shows that similar amounts of APC were precipitated with the anti-Cdc26 antibody.
Figure 2
The APC is phosphorylated in vivo. A, APC phosphorylation is greatest in mitosis. Wild-type (ADR376) and _cdc26_Δ (LH307) were grown overnight in YEP + 2% glucose at 23°C to log phase and then arrested in G1 with alpha factor (1 μg/ml), in S-phase with hydroxyurea (200 mM), or in mitosis with nocodazole (10 μg/ml) for 3 h. Cells were then transferred to phosphate-free CSM + 2% glucose containing 32PO4, and alpha factor, HU, or nocodazole as indicated. After 1 h cells were harvested, lysed, and the APC was immunoprecipitated with anti-Cdc26 antibody. Immunoprecipitates were run on a polyacrylamide gel that was subjected to either autoradiography (top) or Western blotting (bottom). B and C, CDC28-VF, _clb2_Δ, cdc28-1N, and cdc5-1 have reduced APC phosphorylation in vivo. All strains contain pGAL-MPS1. Wild-type (KH153), CDC28-VF (KH181), _clb2_Δ (ADR1606), cdc28-1N (ADR1899), cdc5-1 (JC165), and _cdc26_Δ (ADR2023) were grown overnight in YEP + 2% raffinose at 23°C to log phase, and were then transferred to YEP + 2% galactose for 4 h to arrest the cells in mitosis by Mps1 overexpression. Cells were then transferred to phosphate-free CSM + 2% galactose containing 32PO4, and treated as described in A. In B, cells were arrested by Mps1 overexpression at 23°C, whereas in C cells were arrested at 35°C. In all experiments, the Western blots shown below the autoradiographs illustrate that the same amount of APC was immunoprecipitated in all strains (except for _cdc26_Δ strains, where no APC was precipitated).
Figure 3
The APC is phosphorylated on potential Cdc28 phosphorylation sites. A, All serine/proline (SP) and threonine/proline (TP) sites on Cdc16, Cdc23, and Cdc27 were mutated to alanine/proline (AP). B, Phosphorylation site mutants are resistant to phosphorylation in vivo. All strains contain pGAL-MPS1. Wild-type (KH153), CDC16-6A (ADR1975), CDC23-A-HA (ADR1973), CDC27-5A-HA (ADR1974); and CDC16-6A CDC23-A CDC27-5A (ADR1979) and _cdc26_Δ (ADR2023) were grown in the presence of 32PO4 as described in Fig. 1 B. C, Phosphorylation site mutants are resistant to phosphorylation in vitro. The APC was isolated and phosphorylated in vitro as described in Fig. 1 for: _cdc26_Δ (LH307), CDC23-A (ADR2030), wild-type (ADR376), CDC27-5A (ADR2031), CDC16-6A (ADR2029), and CDC16-6A CDC23-A CDC27-5A (ADR2032).
Figure 4
The APC associates with Cks1-coupled beads. A, The APC from CDC28-VF associates poorly with Cks-coupled beads. Wild-type (ADR477) and CDC28-VF (ADR509) were grown overnight in YEP + 2% glucose at 23°C to log phase and arrested in mitosis with nocodazole (10 μg/ml) for 3 h. Cells were harvested, lysed, and mixed with Cks1-coupled beads. Western blots of the material bound to the Cks1-coupled beads show that the APC and Cdc28/Clb2 bind to the beads. B, Cdc27 phosphorylation can be seen by Western blotting. Wild-type APC, isolated as described in A, was treated either with lambda phosphatase, lambda phosphatase and inhibitors, or inhibitors alone. C, APC association to Cks1-coupled beads changes through the cell cycle. Wild-type (ADR1389) and CDC28-VF (ADR1252) were grown overnight at 30°C in YPD to log phase, arrested in G1 with alpha factor (1 μg/ml), and at t = 0 (alpha factor) the cells were released from the G1 arrest. At t = 75, alpha factor (1.5 μg/ml) was added back to the cultures to rearrest the cells in the next G1. A parallel sample was arrested in mitosis with nocodazole (10 μg/ml). Samples were taken at the indicated times and processed for Western blots (top) or for Cks-coupled bead pulldowns (bottom). The arrow indicates that in wild-type cells when Clb2 levels peak (t = 75), Cdc16 and Cdc27 phosphorylation increases. The bracket indicates that in CDC28-VF cells when Clb2 levels are peaking, Cdc27 phosphorylation decreases. D, An APC-containing Cdc27-5A does not bind to Cks1-coupled beads. The strains in Fig. 3 B and CDC28-VF pGAL-MPS1 (KH181) were grown overnight in YEP + 2% raffinose at 23°C to log phase and then transferred to YEP + 2% galactose for 4 h to arrest the cells in mitosis by Mps1 overexpression. Samples were taken and processed for Western blots (cell lysate), immunoprecipitation with anti-cdc26 antibodies, or Cks1-coupled bead pulldowns as described in Materials and Methods. Equal amounts of lysates were used for the Cks1-coupled bead pulldown and the anti-Cdc26 immunoprecipitation, though a longer exposure is shown for the Cks1-coupled bead pulldown. Clb2 is shown as a loading control. E, Mitotic Cdc28 activity is required for APC phosphorylation. Wild-type (ADR376), CDC28-VF (ADR509), cdc28-1N (ADR483), cdc28-4 (ADR842), _clb2_Δ (ADR313), and cks1-38 (ADR1767) were grown overnight in YEP + 2% glucose at 23°C to log phase and arrested in mitosis with nocodazole (10 μg/ml) for 3.5 h. Samples were taken at the indicated times and processed for Western blots. Clb3 is shown as a loading control.
Figure 5
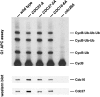
The alanine-substituted APC has normal G1 APC activity. The strains described in Fig. 3 C were grown overnight at 30°C in YEP + 2% glucose to log phase, and arrested in G1 with alpha factor (1 μg/ml) for 3 h. The cells were harvested, lysed, and the APC was immunoprecipitated with anti-Cdc26 antibodies, and the in vitro ubiquitination activity of the immunoprecipitates was measured. The substrate for the in vitro ubiquitination is an iodinated NH2-terminal fragment of sea urchin Cyclin B (CycB). Western blotting of the immunoprecipitates (bottom) shows that equal amounts of Cdc16 and Cdc27 are present in the APC isolated from each of the strains.
Figure 6
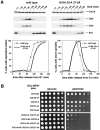
The alanine-substituted APC delays in mitosis and is sensitive to spindle checkpoint-dependent arrest. A, Wild-type (ADR2061) and CDC16-6A CDC23-A CDC27-5A (ADR2064) were grown overnight at 23°C in YPD to mid log phase, arrested in G1 with alpha factor (1 μg/ml), and at t = 0 the cells were released from the G1 arrest. At t = 80, alpha factor (1.5 μg/ml) was added back to the cultures to rearrest the cells in the next G1. Top, Samples were taken at the indicated times and processed for Western blots. Bottom left, Sister chromatid separation was scored by counting the number of fluorescent spots (one or two) of green fluorescent protein (GFP)-lacI bound to 256 tandem repeats of lacO integrated at the LEU2 locus. Bottom right, Spindles were visualized by indirect immunofluorescence of formaldehyde fixed cells probed with an antialpha-tubulin antibody. B, All strains contain pGAL-MPS1. Wild-type (KH153), CDC28-VF (KH181), CDC23-A (ADR1973), CDC27-5A (ADR1974), CDC16-6A (ADR1975), CDC23-A CDC27-5A (ADR1976), CDC16-6A CDC23-A (ADR1977), CDC16-6A CDC27-5A (ADR1978), and CDC16-6A CDC23-A CDC27-5A (ADR1979) were grown to saturation for 2 d in YEP + 2% glucose at 30°C, diluted tenfold, and fourfold serial dilutions were prepared in a multiwell dish and spotted onto YEP + 2% glucose (left) or YEP + 2% galactose (right). The plates were incubated at 30°C for 2 d.
Figure 7
The alanine-substituted APC is defective in Cdc20-dependent APC function. A, Pds1 is stabilized in anaphase in CDC16-6A and CDC27-5A. cdc15-2 GAL-PDS1-HA (ADR1968), cdc15-2 CDC27-5A GAL-PDS1-HA (ADR1999), and cdc15-2 CDC16-6A GAL-PDS1-HA (ADR2003) were grown overnight at 23°C in YEP + 2% raffinose to log phase and shifted to 37°C to arrest the cells in anaphase (raf). When >85% of the cells had reached anaphase (after 4 h, as judged by nuclear division, which was scored by 4′,6-diamidino-2-phenylindole [DAPI] staining), Pds1-HA expression was induced for 1 h by the addition of 2% galactose, and at t = 0, its expression was terminated by the addition of 2% glucose. Samples were taken at the indicated times and processed for Western blots. Clb2 and Cdc27 are shown as a loading controls. B, Cdc20 binding to the APC is impaired in CDC16-6A. cdc15-2 CDC20-myc12 (ADR1790), cdc15-2 CDC27-5A CDC20-myc12 (ADR1987), cdc15-2 CDC20-myc12 CDC16-6A (ADR1990), and _cdc26_Δ CDC20-myc12 (ADR2036) were grown overnight in YEP + 2% glucose at 23°C to log phase and transferred into fresh YEP + 2% glucose at 37°C. When >85% of the cells were arrested in anaphase (4 h, as judged by nuclear division, which was scored by DAPI staining), the cells were harvested, lysed, and the APC was immunoprecipitated with polyclonal anti-Cdc26 antibodies. The amount of Cdc20-myc12 bound to the APC was determined by Western blotting the immunoprecipitates with the 9E10 antibody. Equal amounts of Cdc23 was precipitated with the anti-Cdc26 antibodies (left) and equal amounts of cell lysate were used in the immunoprecipitation (right, cell lysate). _cdc26_Δ, which arrests in metaphase, not anaphase, accumulates high levels of Cdc20 because Cdc20 stability is regulated by the APC (Prinz et al. 1998; Shirayama et al. 1998).
Figure 8
Cdc5 phosphorylates the APC in vitro. A, The APC was isolated from the following strains as described in Fig. 1: Wild-type (ADR376), APC9-HA3 (ADR2042), CDC16-myc6 (K6180), CDC23-HA (SLJ378), CDC27-MBP (ADR1705), and _cdc26_Δ (LH307). The immunoprecipitates were treated with purified His6-HA-Cdc5 and γ[32P]ATP, washed to remove phosphorylated His6-HA-Cdc5, and run on a polyacrylamide gel that was subjected to autoradiography (top) or Western blotting (bottom). The Western blot shows that similar amounts of APC were precipitated with the anti-Cdc26 antibody from all strains except APC9-HA3, which does not fully complement a _apc9_Δ. B, The APC was isolated from wild-type (ADR376), CDC16-6A CDC23-A CDC27-5A (ADR2032), and _cdc26_Δ (LH307), and phosphorylated by purified His6-HA-Cdc5.
Similar articles
- Cdc20 protein contains a destruction-box but, unlike Clb2, its proteolysisis not acutely dependent on the activity of anaphase-promoting complex.
Goh PY, Lim HH, Surana U. Goh PY, et al. Eur J Biochem. 2000 Jan;267(2):434-49. doi: 10.1046/j.1432-1327.2000.01014.x. Eur J Biochem. 2000. PMID: 10632713 - Cdc28 activates exit from mitosis in budding yeast.
Rudner AD, Hardwick KG, Murray AW. Rudner AD, et al. J Cell Biol. 2000 Jun 26;149(7):1361-76. doi: 10.1083/jcb.149.7.1361. J Cell Biol. 2000. PMID: 10871278 Free PMC article. - The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis.
Prinz S, Hwang ES, Visintin R, Amon A. Prinz S, et al. Curr Biol. 1998 Jun 18;8(13):750-60. doi: 10.1016/s0960-9822(98)70298-2. Curr Biol. 1998. PMID: 9651679 - Subunits and substrates of the anaphase-promoting complex.
Peters JM. Peters JM. Exp Cell Res. 1999 May 1;248(2):339-49. doi: 10.1006/excr.1999.4443. Exp Cell Res. 1999. PMID: 10222126 Review. - Control of mitotic transitions by the anaphase-promoting complex.
Fang G, Yu H, Kirschner MW. Fang G, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1583-90. doi: 10.1098/rstb.1999.0502. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582244 Free PMC article. Review.
Cited by
- Filamin a regulates neural progenitor proliferation and cortical size through Wee1-dependent Cdk1 phosphorylation.
Lian G, Lu J, Hu J, Zhang J, Cross SH, Ferland RJ, Sheen VL. Lian G, et al. J Neurosci. 2012 May 30;32(22):7672-84. doi: 10.1523/JNEUROSCI.0894-12.2012. J Neurosci. 2012. PMID: 22649246 Free PMC article. - Panta rhei: the APC/C at steady state.
Primorac I, Musacchio A. Primorac I, et al. J Cell Biol. 2013 Apr 15;201(2):177-89. doi: 10.1083/jcb.201301130. J Cell Biol. 2013. PMID: 23589490 Free PMC article. Review. - CDK Regulation of Meiosis: Lessons from S. cerevisiae and S. pombe.
MacKenzie AM, Lacefield S. MacKenzie AM, et al. Genes (Basel). 2020 Jun 29;11(7):723. doi: 10.3390/genes11070723. Genes (Basel). 2020. PMID: 32610611 Free PMC article. Review. - Molecular mechanism of APC/C activation by mitotic phosphorylation.
Zhang S, Chang L, Alfieri C, Zhang Z, Yang J, Maslen S, Skehel M, Barford D. Zhang S, et al. Nature. 2016 May 12;533(7602):260-264. doi: 10.1038/nature17973. Epub 2016 Apr 27. Nature. 2016. PMID: 27120157 Free PMC article. - Budding yeast PAK kinases regulate mitotic exit by two different mechanisms.
Chiroli E, Fraschini R, Beretta A, Tonelli M, Lucchini G, Piatti S. Chiroli E, et al. J Cell Biol. 2003 Mar 17;160(6):857-74. doi: 10.1083/jcb.200209097. J Cell Biol. 2003. PMID: 12642613 Free PMC article.
References
- Brown N.R., Noble M.E., Endicott J.A., Johnson L.N. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat. Cell Biol. 1999;1:438–443. - PubMed
- Charles J., Jaspersen S.L., Tinker-Kulberg R.L., Hwang L., Szidon A., Morgan D.O. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae . Curr. Biol. 1998;9:497–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous