TPX2, A novel xenopus MAP involved in spindle pole organization - PubMed (original) (raw)
TPX2, A novel xenopus MAP involved in spindle pole organization
T Wittmann et al. J Cell Biol. 2000.
Abstract
TPX2, the targeting protein for Xenopus kinesin-like protein 2 (Xklp2), was identified as a microtubule-associated protein that mediates the binding of the COOH-terminal domain of Xklp2 to microtubules (Wittmann, T., H. Boleti, C. Antony, E. Karsenti, and I. Vernos. 1998. J. Cell Biol. 143:673-685). Here, we report the cloning and functional characterization of Xenopus TPX2. TPX2 is a novel, basic 82.4-kD protein that is phosphorylated during mitosis in a microtubule-dependent way. TPX2 is nuclear during interphase and becomes localized to spindle poles in mitosis. Spindle pole localization of TPX2 requires the activity of the dynein-dynactin complex. In late anaphase TPX2 becomes relocalized from the spindle poles to the midbody. TPX2 is highly homologous to a human protein of unknown function and thus defines a new family of vertebrate spindle pole components. We investigated the function of TPX2 using spindle assembly in Xenopus egg extracts. Immunodepletion of TPX2 from mitotic egg extracts resulted in bipolar structures with disintegrating poles and a decreased microtubule density. Addition of an excess of TPX2 to spindle assembly reactions gave rise to monopolar structures with abnormally enlarged poles. We conclude that, in addition to its function in targeting Xklp2 to microtubule minus ends during mitosis, TPX2 also participates in the organization of spindle poles.
Figures
Figure 1
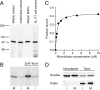
Molecular cloning of TPX2. (A) Northern blot of total Xenopus egg RNA hybridized with a PCR fragment obtained in a reaction with degenerate oligonucleotides designed according to TPX2 peptide sequences. The amount of RNA loaded per lane is indicated on top. The migration position of the 28S rRNA (4,115 nucleotides) and the 18S rRNA (1,826 nucleotides) is shown on the left. The probe recognizes a unique transcript of 3.0–3.5 kb. (B) Amino acid sequence of TPX2. Peptide sequences obtained by mass spectrometry are underlined. Differences between the predicted protein sequence and the peptide sequences are indicated by dotted underlines. Putative cdc2 kinase sites, (S/T)PX(K/R), are shaded in dark gray and MAP kinase phosphorylation sites, PX(S/T)P, are shaded in light gray, nuclear localization signals are shown in bold and the predicted coiled-coil regions are in italics. The complete cDNA sequence of Xenopus laevis TPX2 is available from GenBank/EMBL/DDBJ under accession number AF244546. (C) Sequence comparison of a region from TPX2 (corresponding to amino acids 297–356) with the human protein fls353 (amino acids 328–387) and predicted protein sequences from ESTs from mouse, chicken and zebrafish (these data are available from GenBank/EMBL/DDBJ under accession number AI415194, AJ394859, and AI883915). Conserved residues are shaded in gray and boxes indicate the highly conserved cdc2 and MAP kinase phosphorylation sites within this region.
Figure 2
TPX2 is phosphorylated in mitotic egg extract and binds to microtubules with high affinity. (A) Immunoblot of low-speed Xenopus egg extracts, mitotic microtubule-associated proteins purified as described (Wittmann et al. 1998) and XL177 SDS lysate probed with affinity-purified anti-TPX2N. The molecular mass of marker proteins is indicated on the left. (B) Phosphorylation of TPX2 in mitotic, M, and interphase, I, egg extract incubated in the presence of γ-[32P]ATP. Taxol was added to 2 μM and the extracts were incubated for 30 min at 20°C. The immunoprecipitates were analyzed on a 6% SDS-PAGE and subjected to autoradiography. TPX2 is phosphorylated in mitotic extract and hyperphosphorylated upon assembly of microtubules. (C) Microtubule sedimentation assay of GFP-TPX2 with pure microtubules. The fraction of GFP-TPX2 bound to microtubules is plotted versus the microtubule concentration. The solid line represents the hyperbolic curve fit. (D) Microtubule sedimentation assay from crude extract. Interphase, I, and mitotic, M, extract were supplemented with either 20 μM nocodazole or 2 μM taxol, incubated for 20 min at 20°C and centrifuged through a 10% sucrose cushion. The immunoblots show the amount of TPX2 present in equivalent amounts of the soluble and pelleted fraction.
Figure 3
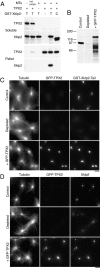
TPX2 is required for the binding of Xklp2 to microtubules. (A) Microtubule sedimentation assay. Pure prepolymerized microtubules (1 μM) were mixed with bacterially expressed TPX2 and GST-Xklp2-Tail (both 0.1–0.2 μM), T, or GST-Xklp2-CDel2, C (Wittmann et al. 1998), as indicated on top, incubated for 20 min at 20°C and centrifuged through a 10% sucrose cushion. Equivalent amounts of the soluble and pelleted fraction were analyzed by immunoblot probed with anti-TPX2N or anti-Xklp2 antibodies as indicated. GST-Xklp2-Tail is only recovered in the pellet in the presence of both microtubules and TPX2. (B) Immunodepletion of TPX2 and GFP-TPX2 addback. 1-μl aliquots of extract were analyzed by immunoblot and probed with anti-TPX2N. The molecular mass of marker proteins is indicated on the left. Additional bands in the addback lane are due to degradation products of GFP-TPX2. (C) Mitotic asters assembled for 30 min at 20°C in the presence of 5% DMSO, 1 μM GST-Xklp2-Tail, and rhodamine-labeled tubulin in control- and TPX2-depleted extract supplemented with bacterially expressed GFP-TPX2. The asters were sedimented onto coverslips and stained with an anti-GST and an Alexa350-conjugated anti–rabbit antibody. (D) Mitotic asters assembled in the presence of Cy5-labeled tubulin as in C but without GST-Xklp2-Tail added. The asters were stained with an anti-Xklp2 and a rhodamine-conjugated anti–rabbit antibody. Bars, 10 μm.
Figure 4
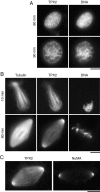
(A) Immunolocalization of TPX2 throughout the cell cycle. XL177 Xenopus tissue culture cells were fixed in cold methanol and stained with 0.6 μg/ml affinity-purified anti-TPX2N, monoclonal anti-tubulin, and Hoechst 33258 followed by FITC-labeled anti–mouse and TRITC-labeled anti–rabbit antibodies. Images represent single confocal slices. The same staining pattern was observed with anti-TPX2 or when the cells were fixed with glutaraldehyde. TPX2 staining appears at the end of prophase, persists at the spindle poles throughout mitosis until late anaphase. It then relocalizes to the midbody where it disappears in late telophase. (B) Live images of a mitotic XL177 cell expressing low amounts of GFP-TPX2. Note the nuclear fluorescence in telophase. The elapsed time is indicated in the upper right corner. Supplemental video is viewable at http://www.jcb.org/cgi/content/full/149/7/1405/DC1\. Bars, 10 μm.
Figure 5
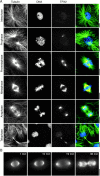
Immunolocalization of TPX2 in Xenopus egg extract. (A) Sperm nuclei were incubated in extract released into interphase by the addition of calcium for the indicated time, fixed and sedimented onto coverslips. (B) Mitotic spindles assembled in the presence of rhodamine-labeled tubulin in cycled CSF-extract. Reactions were fixed at the indicated times after reentry into mitosis and sedimented onto coverslips. Spindles and nuclei were stained with 3 μg/ml anti-TPX2N, a Cy2-labeled anti–rabbit antibody and Hoechst 33258. TPX2 localizes to a branched structure in interphase nuclei and migrates to the spindle poles in mitosis. (C) Mitotic spindles centrifuged onto coverslips, postfixed in cold methanol and stained for either TPX2 or NuMA. Images were taken under identical conditions. Bars, 10 μm.
Figure 6
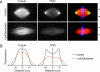
The dynein–dynactin complex is required for the localization of TPX2 to spindle poles. (A) Recombinant p50/dynamitin was added to cycled spindle reactions at ∼0.7 mg/ml. The spindles were sedimented onto coverslips and stained with anti-TPX2N and a Cy2-conjugated anti–rabbit antibody. In the overlay, also the DNA visualized by Hoechst 33258 staining is shown in blue. Fluorescence intensity of the TPX2 staining and the rhodamine-labeled tubulin was measured in a 100-pixel wide stripe (width is indicated by arrowheads on the right) along the spindle axis. (B) The average fluorescence intensity of eleven spindles with (red) and without (black) p50/dynamitin added was plotted against the distance from the metaphase plate.
Figure 8
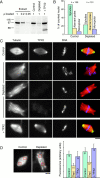
Depletion of TPX2 affects spindle pole structure. (A) Immunoblot probed with anti-TPX2N demonstrating the depletion efficiency. 1-, 0.2-, and 0.05-μl samples of mitotic Xenopus egg extract are compared with 1 μl of control- and TPX2-depleted extract supplemented with bacterially expressed TPX2. The molecular mass of marker proteins is indicated on the left. (B) Quantification of structures observed in control- and TPX2-depleted extract 1 h after cycling the extract back into mitosis. The number of quantified structures is indicated on top. (C) Representative bipolar structures observed in control- and TPX2-depleted extract supplemented with recombinant TPX2. Cycled spindles were assembled in the presence of rhodamine-labeled tubulin, fixed, centrifuged onto coverslips, and stained with anti-TPX2N and Hoechst 33258. (D) Quantification of the microtubule density in bipolar structures assembled in control- and TPX2-depleted extract supplemented with recombinant TPX2 from two independent experiments. The red boxes indicate representative areas in which the rhodamine fluorescence intensity was measured. The number of structures analyzed is shown on top of the columns. Error bars indicate the standard deviation. Bars, 10 μm.
Figure 7
Immunoprecipitation of TPX2 from egg extract. (A) Immunoprecipitated proteins using a control rabbit IgG or anti-TPX2N were eluted with a high-salt buffer and proteins remaining on the beads or recovered in the eluate were analyzed on a Coomassie-stained SDS-PAGE. (B) GST-TPX2 was added to mitotic egg extract, incubated for 30 min at 20°C and immunoprecipitated with an anti-GST antibody. For both gels, the molecular mass of marker proteins is indicated on the left. A polypeptide of ∼90 kD (arrowhead) is specifically immunoprecipitated together with the endogenous TPX2 or with GST-TPX2.
Figure 9
Cycled spindle assembly in Xklp2- and TPX2-depleted extract. (A) Immunoblot of 1-μl samples of control-, TPX2-, Xklp2-, and double-depleted extract compared with 1, 0.2, 0.05, and 0.02 μl of mitotic egg extract and probed for Xklp2 and TPX2 as indicated. (B) Quantification of structures observed under the various depletion conditions. The number of quantified structures is indicated on top. (C) Representative bipolar structures observed in Xklp2- and double-depleted extract. Cycled spindles were assembled in the presence of rhodamine-labeled tubulin, fixed, centrifuged onto coverslips and stained with anti-TPX2N and Hoechst 33258. (D) TPX2-depleted structure stained for NuMA. Bars, 10 μm.
Figure 10
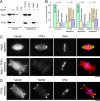
An excess of TPX2 prevents the formation of bipolar spindles. (A) Representative structures observed in control and TPX2-supplemented reactions. Cycled spindles were assembled in the presence of rhodamine-labeled tubulin, fixed, centrifuged onto coverslips, and stained with anti-TPX2N and Hoechst 33258. (B) Quantification of structures observed in spindle assembly reactions supplemented with either control buffer or a 5–10-fold molar excess of TPX2. The number of quantified structures is indicated on top. (C) Quantification of the microtubule density in bipolar control spindles and in monopolar structures in TPX2-supplemented reactions. Error bars indicate the standard deviation (n = 20). (D) Monopolar structure in the presence of excess TPX2 stained for NuMA. Bars, 10 μm.
Similar articles
- Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein.
Wittmann T, Boleti H, Antony C, Karsenti E, Vernos I. Wittmann T, et al. J Cell Biol. 1998 Nov 2;143(3):673-85. doi: 10.1083/jcb.143.3.673. J Cell Biol. 1998. PMID: 9813089 Free PMC article. - Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts.
Brunet S, Sardon T, Zimmerman T, Wittmann T, Pepperkok R, Karsenti E, Vernos I. Brunet S, et al. Mol Biol Cell. 2004 Dec;15(12):5318-28. doi: 10.1091/mbc.e04-05-0385. Epub 2004 Sep 22. Mol Biol Cell. 2004. PMID: 15385625 Free PMC article. - Spindle pole regulation by a discrete Eg5-interacting domain in TPX2.
Eckerdt F, Eyers PA, Lewellyn AL, Prigent C, Maller JL. Eckerdt F, et al. Curr Biol. 2008 Apr 8;18(7):519-25. doi: 10.1016/j.cub.2008.02.077. Epub 2008 Mar 27. Curr Biol. 2008. PMID: 18372177 Free PMC article. - Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.
Sun QY, Schatten H. Sun QY, et al. Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review. - Mitotic motors in Saccharomyces cerevisiae.
Hildebrandt ER, Hoyt MA. Hildebrandt ER, et al. Biochim Biophys Acta. 2000 Mar 17;1496(1):99-116. doi: 10.1016/s0167-4889(00)00012-4. Biochim Biophys Acta. 2000. PMID: 10722880 Review.
Cited by
- Target protein for Xklp2 (TPX2), a microtubule-related protein, contributes to malignant phenotype in bladder carcinoma.
Yan L, Li S, Xu C, Zhao X, Hao B, Li H, Qiao B. Yan L, et al. Tumour Biol. 2013 Dec;34(6):4089-100. doi: 10.1007/s13277-013-1000-z. Epub 2013 Jul 20. Tumour Biol. 2013. PMID: 23873098 - Comprehensive Analysis of the Oncogenic Role of Targeting Protein for Xklp2 (TPX2) in Human Malignancies.
Shao T, Jiang X, Bao G, Li C, Guo C. Shao T, et al. Dis Markers. 2022 Oct 18;2022:7571066. doi: 10.1155/2022/7571066. eCollection 2022. Dis Markers. 2022. PMID: 36304254 Free PMC article. - Prognostic and clinical value of Targeting protein for Xenopus kinesin-like protein 2 in patients with gastrointestinal tract cancers: A meta-analysis.
Liu W, Xu J, Zhang C. Liu W, et al. Medicine (Baltimore). 2018 Nov;97(46):e13303. doi: 10.1097/MD.0000000000013303. Medicine (Baltimore). 2018. PMID: 30431618 Free PMC article. Review. - How Microtubules Build the Spindle Branch by Branch.
Travis SM, Mahon BP, Petry S. Travis SM, et al. Annu Rev Cell Dev Biol. 2022 Oct 6;38:1-23. doi: 10.1146/annurev-cellbio-120420-114559. Epub 2022 Jun 27. Annu Rev Cell Dev Biol. 2022. PMID: 35759800 Free PMC article. Review. - Mechanisms of plant spindle formation.
Zhang H, Dawe RK. Zhang H, et al. Chromosome Res. 2011 Apr;19(3):335-44. doi: 10.1007/s10577-011-9190-y. Chromosome Res. 2011. PMID: 21424324 Review.
References
- Andersen S.S.L. Spindle assembly and the art of regulating microtubule dynamics by MAPs and stathmin/Op18. Trends Cell Biol. 2000;10:261–267. - PubMed
- Ausubel F., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Short Protocols in Molecular Biology. John Wiley & Sons, Inc; New York: 1995.
- Boleti H., Karsenti E., Vernos I. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous