Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity - PubMed (original) (raw)
Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity
M Kruse et al. J Virol. 2000 Aug.
Abstract
Mature dendritic cells (DC) are the most potent antigen-presenting cells within the entire immune system. Interference with the function of these cells therefore constitutes a very powerful mechanism for viruses to escape immune responses. Several members of the Herpesviridae family have provided examples of such escape strategies, including interference with antigen presentation and production of homologous cytokines. In this study we investigated the infection of mature DC with herpes simplex virus type 1 (HSV-1) and the way in which infection alters the phenotype and function of mature DC. Interestingly, the T-cell-stimulatory capacity of these DC was strongly impaired. Furthermore, we demonstrated that HSV-1 leads to the specific degradation of CD83, a cell surface molecule which is specifically upregulated during DC maturation. These data indicate that HSV-1 has developed yet another novel mechanism to escape immune responses.
Figures
FIG. 1
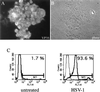
(A) HSV-1 infection induces CD83 downregulation on mature DC. Mature DC were inoculated either with infectious HSV-1 at a MOI of 1 or with UV-inactivated virus and analyzed by FACS analysis after 0, 4, and 24 h. A strong downregulation of cell surface expression of CD83 was observed (left panel). No such effects could be observed when DC were left untreated or inoculated with UV-inactivated HSV-1. In contrast, the expression of MHC class II molecules was not dramatically affected by the HSV-1 infection (right panel). (B) Time course of HSV-1-induced CD83 downregulation. At 10 h after the HSV-1 infection (MOI = 1), a dramatic reduction of the CD83 cell surface expression is already observed. (C) CD83 downregulation is HSV-1 titer dependent. Mature DC were infected with HSV-1 at different MOIs (0.01, 0.1, and 1) and analyzed after 24 h by FACS analysis. The viral infection rate significantly influences the CD83 expression.
FIG. 2
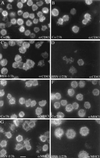
Efficient infection of mature DC by HSV-1. Mature DC were infected with HSV-1 at an MOI of 1 and analyzed after 10 h by intracellular FACS staining or by indirect immunofluorescence using HSV-1 VP16-specific antibody. (A and B) VP16 staining (A) and phase-contrast microscopy (B) of infected DC. (C) More than 90% of the mature DC were infected after this time.
FIG. 3
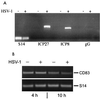
RT-PCR analyses of HSV-1- and CD83-specific mRNA. (A) Total cellular RNA was isolated after 24 h and reverse transcribed from uninfected and HSV-1 (MOI = 1)-infected mature DC. HSV-1-specific PCR primers were used to amplify transcripts from immediate-early (ICP27), early (ICP8), and late (gG) gene transcripts. Amplification of ribosomal protein S14-specific sequences served as an internal control. (B) RT-PCR of CD83-specific mRNA. No difference in the CD83 signals between mock- and HSV-1-infected samples after 4 and 10 h postinfection was detected.
FIG. 4
HSV-1-infected mature DC do not produce infectious virus. Mature DC were infected with HSV-1 at a MOI of 0.01, 0.1, or 1. Cell supernatants were analyzed using the RITA-plaque test after 24 h to determine the amount of infectious particles generated during the infection. No or only low levels of viral particles were detected in supernatant derived from HSV-1-infected DC. In sharp contrast, Vero cells inoculated at a MOI of 0.01 generated 106 virus particles per ml (control). Note the logarithmic scale of the y axis.
FIG. 5
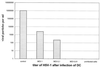
HSV-1 infection decreases the ability of mature DC to induce an allogeneic T-cell response. (A) DC were inoculated with either infectious virus or UV-inactivated virus or left untreated, cocultured with allogeneic T cells for 4 days, and pulsed with [3H]thymidine for 8 h, and the cell supernatants were analyzed. DC derived from noninfected (□) or UV-inactivated HSV-1 (●) induced a strong allostimulatory reaction in the primary allogeneic MLR. In contrast, cells derived from HSV-1-infected DC (■) showed a strongly reduced ability to induce T-cell proliferation, particularly at a T-cell-to-DC ratio of ≤200:1 (MOI = 1). (B) Increasing numbers of uninfected or HSV-1-infected DC were added to 100 HSV-1-infected DC and subsequently analyzed for their allostimulatory capacity. Only when a large excess of infected DC were added (ratio of infected to uninfected DC = 66:1) was an interference with the allostimulation detectable.
FIG. 6
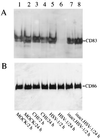
HSV-1 infection induces CD83 protein degradation in mature DC. (A) Total cellular protein extracts (2- and 24-h samples) from DC which were inoculated either with infectious virus (MOI = 1) or with UV-inactivated virus, treated with CHI, or left untreated were resolved using SDS-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and probed with a CD83-specific monoclonal antibody. (B) The blot shown in panel A was reprobed using a CD86-specific antibody.
FIG. 7
HSV-1 infection strongly affects the expression of CD83 in mature DC. Mature DC were either inoculated for 1 h with HSV-1 (MOI = 1) or left untreated. After an incubation period of 3 h (A and C) or 23 h (B and D), the cells were fixed and analyzed by indirect CD83 immunofluorescence. After 3 h, the infected DC showed only a slight change in their CD83 surface expression (C), but a dramatic reduction of the CD83 signal intensity was observed after 23 h (D). The same cell population was also analyzed using an MHC class I-specific antibody (E to H). These analyses did not reveal major differences between the different cell populations. Bar, 20 μm.
FIG. 8
HSV-1 leads to CD83 degradation. The p3CD83 expression plasmid DNA was microinjected into the nuclei of Vero cells, and the cells were cultured for further 20 h. They were then either left untreated or infected with HSV-1 (MOI = 1) prior to a further incubation period of 7 h. The cells were subsequently analyzed by double immunofluorescence microscopy using antibodies directed against CD83 (A and C) and VP16 (B and D). (A) Noninfected Vero cells showed a characteristic CD83 staining. (C) In contrast, HSV-1 infection affected the normal CD83 distribution, leading to accumulated intracellular structures. (D) Indirect VP16 immune fluorescence served as control for the successful virus infection. More infected cells show less CD83 expression. Compare cells labeled # and ∗ in panels C and D. Bar, 20 μm.
FIG. 9
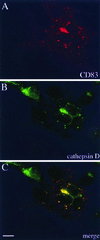
Subcellular localization of CD83. (A and B) To define the cellular CD83 localization after HSV-1 infection, cells were stained with antibodies against CD83 (A) and cathepsin D (B), which showed a similar intracellular distribution. (C) Merging of the images in panels A and B indicates that CD83 accumulates in lysosomal compartments. Bar, 20 μm.
Similar articles
- The interaction between dendritic cells and herpes simplex virus-1.
Kobelt D, Lechmann M, Steinkasserer A. Kobelt D, et al. Curr Top Microbiol Immunol. 2003;276:145-61. doi: 10.1007/978-3-662-06508-2_7. Curr Top Microbiol Immunol. 2003. PMID: 12797447 Review. - L Particles Transmit Viral Proteins from Herpes Simplex Virus 1-Infected Mature Dendritic Cells to Uninfected Bystander Cells, Inducing CD83 Downmodulation.
Heilingloh CS, Kummer M, Mühl-Zürbes P, Drassner C, Daniel C, Klewer M, Steinkasserer A. Heilingloh CS, et al. J Virol. 2015 Nov;89(21):11046-55. doi: 10.1128/JVI.01517-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311871 Free PMC article. - Herpes simplex virus type 1 ICP0 induces CD83 degradation in mature dendritic cells independent of its E3 ubiquitin ligase function.
Heilingloh CS, Mühl-Zürbes P, Steinkasserer A, Kummer M. Heilingloh CS, et al. J Gen Virol. 2014 Jun;95(Pt 6):1366-1375. doi: 10.1099/vir.0.062810-0. Epub 2014 Mar 18. J Gen Virol. 2014. PMID: 24643878 - Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity.
Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, Coffin RS, Katz DR, Chain B. Pollara G, et al. J Infect Dis. 2003 Jan 15;187(2):165-78. doi: 10.1086/367675. Epub 2003 Jan 6. J Infect Dis. 2003. PMID: 12552441 - Role of CD83 in the immunomodulation of dendritic cells.
Lechmann M, Zinser E, Golka A, Steinkasserer A. Lechmann M, et al. Int Arch Allergy Immunol. 2002 Oct;129(2):113-8. doi: 10.1159/000065883. Int Arch Allergy Immunol. 2002. PMID: 12403928 Review.
Cited by
- HSV-1 Modulates IL-6 Receptor Expression on Human Dendritic Cells.
Birzer A, Krawczyk A, Draßner C, Kuhnt C, Mühl-Zürbes P, Heilingloh CS, Steinkasserer A, Popella L. Birzer A, et al. Front Immunol. 2020 Aug 26;11:1970. doi: 10.3389/fimmu.2020.01970. eCollection 2020. Front Immunol. 2020. PMID: 32983130 Free PMC article. - Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs.
Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K. Yoneyama H, et al. J Exp Med. 2005 Aug 1;202(3):425-35. doi: 10.1084/jem.20041961. J Exp Med. 2005. PMID: 16061729 Free PMC article. - Loss of mandibular lymph node integrity is associated with an increase in sensitivity to HSV-1 infection in CD118-deficient mice.
Conrady CD, Thapa M, Wuest T, Carr DJ. Conrady CD, et al. J Immunol. 2009 Mar 15;182(6):3678-87. doi: 10.4049/jimmunol.0803878. J Immunol. 2009. PMID: 19265146 Free PMC article. - The tug-of-war between dendritic cells and human chronic viruses.
Rahman S, Khan ZK, Jain P. Rahman S, et al. Int Rev Immunol. 2011 Oct-Dec;30(5-6):341-65. doi: 10.3109/08830185.2011.561506. Int Rev Immunol. 2011. PMID: 22053973 Free PMC article. Review. - Crosstalk Between Epithelial Cells, Neurons and Immune Mediators in HSV-1 Skin Infection.
Duarte LF, Reyes A, Farías MA, Riedel CA, Bueno SM, Kalergis AM, González PA. Duarte LF, et al. Front Immunol. 2021 May 3;12:662234. doi: 10.3389/fimmu.2021.662234. eCollection 2021. Front Immunol. 2021. PMID: 34012447 Free PMC article. Review.
References
- Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
- Assenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of IL-10 in interferon-gamma and in interleukin-4 expressing cells. Eur J Immunol. 1994;24:1097–1101. - PubMed
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources