Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages - PubMed (original) (raw)
Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages
D M Ward et al. Mol Biol Cell. 2000 Jul.
Free PMC article
Abstract
Endocytosis in alveolar macrophages can be reversibly inhibited, permitting the isolation of endocytic vesicles at defined stages of maturation. Using an in vitro fusion assay, we determined that each isolated endosome population was capable of homotypic fusion. All vesicle populations were also capable of heterotypic fusion in a temporally specific manner; early endosomes, isolated 4 min after internalization, could fuse with endosomes isolated 8 min after internalization but not with 12-min endosomes or lysosomes. Lysosomes fuse with 12-min endosomes but not with earlier endosomes. Using homogenous populations of endosomes, we have identified Syntaxin 7 as a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) required for late endosome-lysosome and homotypic lysosome fusion in vitro. A bacterially expressed human Syntaxin 7 lacking the transmembrane domain inhibited homotypic late endosome and lysosome fusion as well as heterotypic late endosome-lysosome fusion. Affinity-purified antibodies directed against Syntaxin 7 also inhibited lysosome fusion in vitro but had no affect on homotypic early endosome fusion. Previous work suggested that human VAMP-7 (vesicle-associated membrane protein-7) was a SNARE required for late endosome-lysosome fusion. A bacterially expressed human VAMP-7 lacking the transmembrane domain inhibited both late endosome-lysosome fusion and homotypic lysosome fusion in vitro. These studies indicate that: 1) fusion along the endocytic pathway is a highly regulated process, and 2) two SNARE molecules, Syntaxin 7 and human VAMP-7, are involved in fusion of vesicles in the late endocytic pathway in alveolar macrophages.
Figures
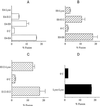
Figure 1
Endosome and lysosome (Lyso) fusion in vitro. Isolated vesicle populations were combined in the in vitro fusion system as described in MATERIALS AND METHODS. Cells were incubated in hypo-K+ buffer for 30 min to stop endocytosis. Cells were then incubated in iso-Na+ buffers containing either b-HRP or avidin for 4 min, washed, and chased in hypo-K+ buffer for additional times. E4 (A) represents endosomes isolated from cells that had internalized avidin or b-HRP for 4 min. E8 (B) represents endosomes isolated from cells that had internalized ligand for 4 min followed by 4 min of chase time. E12 (C) represents endosomes isolated from cells that had internalized ligand for 4 min followed by 8 min of chase time. Lysosomes (D) were isolated from cells allowed to internalize ligand for 30 min followed by a 60-min chase period. Lysosome fusion with E4, E8, and E12 was measured (A–C), as was lysosome–lysosome fusion (D). The percentage of in vitro fusion was calculated with the use of the maximum avidin–b-HRP complex formation in the absence of biotinylated insulin as the denominator.
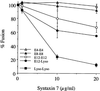
Figure 2
Syntaxin 7 concentration-dependent inhibition of homotypic vesicle fusion in vitro. Purified endosomes (E4, E8, and E12) and lysosomes (Lyso) containing avidin or b-HRP were incubated in the in vitro fusion system. Specified amounts (0–20 μg/ml) of purified recombinant Syntaxin 7, minus the transmembrane domain, were added before incubation at 37°C. The data are expressed as the percentage of fusion measured, where 100% represents the maximum fusion observed.
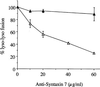
Figure 3
Anti-Syntaxin 7 inhibition of homotypic lysosome (Lyso) fusion in vitro. Purified lysosomes containing avidin or b-HRP were incubated in the in vitro fusion system containing affinity-purified rabbit polyclonal anti-Syntaxin 7 antibody (▵) or preimmune serum (▴). The data are expressed as the percentage of fusion, where 100% represents the maximum fusion observed.
Figure 4
Western analysis of GST-Syntaxin 7 binding to endosomes and lysosomes in vitro. Purified lysosomes or endosomes (0.5 mg/ml) were incubated in the in vitro fusion system in the presence of 20 μg/ml GST-Syntaxin 7. After incubation at 37°C, fusion was assessed as described in MATERIALS AND METHODS. Samples of endosomes or lysosomes from the fusion assay were pelleted and washed with fusion buffer. Vesicle pellets were solubilized by the addition of 0.05% Triton X-100 containing 10 mM Tris-HCl, pH 7.5, and 50 mM NaCl at 0°C for 30 min followed by centrifugation at 40,000 × g for 30 min. Supernatants were incubated with glutathione–agarose beads at 0°C for 2 h or overnight, beads were pelleted and washed, and GST protein was eluted with 20 mM glutathione according to the Pierce protocol. Eluted samples were run on 4–20% SDS-PAGE, and Western analysis was performed with the use of affinity-purified rabbit anti-Syntaxin 7 (1:1000) as the primary antibody followed by HRP-conjugated goat anti-rabbit secondary antibody (1:10,000). A represents samples from lysosomes and B represents endosomal samples. Lanes A represent purified GST-Syntaxin 7 eluted from glutathione–agarose. Lanes B represent the supernatant from pelleted fusion reactions. Lanes C represent lysates after incubation with glutathione–agarose. Lanes D represent a glutathione–agarose bead wash after lysate incubation. Lanes E represent glutathione eluates from the lysosome or endosome glutathione–agarose beads. The arrow represents GST-Syntaxin 7 eluted from only purified lysosomes.
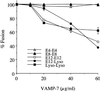
Figure 5
Addition of VAMP-7 to in vitro homotypic/heterotypic endosome and lysosome (Lyso) fusion. Specified amounts of recombinant VAMP-7 (minus the transmembrane domain) were added to the in vitro fusion reaction of E4–E4, E8–E8, E12–E12, Lyso–Lyso (all homotypic), and E12–Lyso (heterotypic). The data are expressed as the percentage of fusion, with 100% fusion representing the maximum fusion observed for each vesicle population.
Similar articles
- Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and Is required for late endosome-lysosome fusion.
Mullock BM, Smith CW, Ihrke G, Bright NA, Lindsay M, Parkinson EJ, Brooks DA, Parton RG, James DE, Luzio JP, Piper RC. Mullock BM, et al. Mol Biol Cell. 2000 Sep;11(9):3137-53. doi: 10.1091/mbc.11.9.3137. Mol Biol Cell. 2000. PMID: 10982406 Free PMC article. - The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes.
Antonin W, Holroyd C, Tikkanen R, Höning S, Jahn R. Antonin W, et al. Mol Biol Cell. 2000 Oct;11(10):3289-98. doi: 10.1091/mbc.11.10.3289. Mol Biol Cell. 2000. PMID: 11029036 Free PMC article. - Hrs regulates early endosome fusion by inhibiting formation of an endosomal SNARE complex.
Sun W, Yan Q, Vida TA, Bean AJ. Sun W, et al. J Cell Biol. 2003 Jul 7;162(1):125-37. doi: 10.1083/jcb.200302083. J Cell Biol. 2003. PMID: 12847087 Free PMC article. - The delivery of endocytosed cargo to lysosomes.
Luzio JP, Parkinson MD, Gray SR, Bright NA. Luzio JP, et al. Biochem Soc Trans. 2009 Oct;37(Pt 5):1019-21. doi: 10.1042/BST0371019. Biochem Soc Trans. 2009. PMID: 19754443 Review. - Membrane traffic to and from lysosomes.
Luzio JP, Pryor PR, Gray SR, Gratian MJ, Piper RC, Bright NA. Luzio JP, et al. Biochem Soc Symp. 2005;(72):77-86. doi: 10.1042/bss0720077. Biochem Soc Symp. 2005. PMID: 15649132 Review.
Cited by
- High-throughput identification of IMCD proteins using LC-MS/MS.
Pisitkun T, Bieniek J, Tchapyjnikov D, Wang G, Wu WW, Shen RF, Knepper MA. Pisitkun T, et al. Physiol Genomics. 2006 Apr 13;25(2):263-76. doi: 10.1152/physiolgenomics.00214.2005. Epub 2006 Jan 31. Physiol Genomics. 2006. PMID: 16449382 Free PMC article. - Afipia felis induces uptake by macrophages directly into a nonendocytic compartment.
Luhrmann A, Streker K, Schüttfort A, Daniels JJ, Haas A. Luhrmann A, et al. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7271-6. doi: 10.1073/pnas.121190398. Epub 2001 Jun 12. Proc Natl Acad Sci U S A. 2001. PMID: 11404461 Free PMC article. - Mammalian ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain.
Hasegawa H, Zinsser S, Rhee Y, Vik-Mo EO, Davanger S, Hay JC. Hasegawa H, et al. Mol Biol Cell. 2003 Feb;14(2):698-720. doi: 10.1091/mbc.e02-09-0556. Mol Biol Cell. 2003. PMID: 12589064 Free PMC article. - Identification of Synaptic DGKθ Interactors That Stimulate DGKθ Activity.
Barber CN, Goldschmidt HL, Ma Q, Devine LR, Cole RN, Huganir RL, Raben DM. Barber CN, et al. Front Synaptic Neurosci. 2022 Apr 27;14:855673. doi: 10.3389/fnsyn.2022.855673. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35573662 Free PMC article. - A VAMP7/Vti1a SNARE complex distinguishes a non-conventional traffic route to the cell surface used by KChIP1 and Kv4 potassium channels.
Flowerdew SE, Burgoyne RD. Flowerdew SE, et al. Biochem J. 2009 Mar 15;418(3):529-40. doi: 10.1042/BJ20081736. Biochem J. 2009. PMID: 19138172 Free PMC article.
References
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL026922/HL/NHLBI NIH HHS/United States
- HL26922/HL/NHLBI NIH HHS/United States
- T32 DK007115/DK/NIDDK NIH HHS/United States
- T32DK07115/DK/NIDDK NIH HHS/United States
- R01 HL026922/HL/NHLBI NIH HHS/United States
- NS36670/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases