Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export - PubMed (original) (raw)
Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export
T Guan et al. Mol Cell Biol. 2000 Aug.
Abstract
We present here a detailed analysis of a rat polypeptide termed Nup50 (formerly NPAP60) that was previously found to be associated with the nuclear pore complex (F. Fan et al., Genomics 40:444-453, 1997). We have found that Nup50 (and/or a related 70-kDa polypeptide) is present in numerous rat cells and tissues. By immunofluorescence microscopy, Nup50 was found to be highly concentrated at the nuclear envelope of rat liver nuclei, whereas in cultured NRK cells it also is abundant in intranuclear regions. On the basis of immunogold electron microscopy of both rat liver nuclear envelopes and NRK cells, we determined that Nup50 is specifically localized in the nucleoplasmic fibrils of the pore complex. Microinjection of anti-Nup50 antibodies into the nucleus of NRK cells resulted in strong inhibition of nuclear export of a protein containing a leucine-rich nuclear export sequence, whereas nuclear import of a protein containing a classical nuclear localization sequence was unaffected. Correspondingly, CRM1, the export receptor for leucine-rich export sequences, directly bound to a fragment of Nup50 in vitro, whereas several other import and export receptors did not significantly interact with this fragment. Taken together, our data indicate that Nup50 has a direct role in nuclear protein export and probably serves as a binding site on the nuclear side of the pore complex for export receptor-cargo complexes.
Figures
FIG. 1
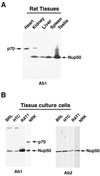
Immunoblot analysis of Nup50 antigens in various cell lines and tissues from rats. Samples of various rat tissues (A) or cultured cell lines (B), as indicated, were analyzed by immunoblotting with antibody 1 (panel A and the left part part of panel B) and antibody 2 (the right part of panel B). The positions of Nup50 and the major ∼70-kDa antigen that is recognized by antibody 1 are indicated.
FIG. 2
MALDI mass spectrometry analysis of Nup50 and p70 antigens of NRK cells. Gel-purified Nup50 and p70 bands from anti-Nup50 immunoprecipitates of NRK cells were digested with trypsin, and the products were analyzed by MALDI time-of-flight mass spectrometry. Short bars indicate peptides smaller than 3,000 Da from each band that match (within experimental accuracy) the predicted mass of peptides from the deduced cDNA sequence of rat Nup50. The total regions of the predicted sequence of Nup50 that are represented by peptides obtained in the MALDI analysis are indicated by filled regions of the top bar designating the linear sequence of Nup50.
FIG. 3
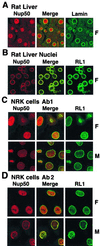
Immunofluorescent staining of rat liver nuclei and NRK cells with anti-Nup50 antibodies. Rat liver cryosections (A), isolated rat liver nuclei (B), or NRK cells (C and D) were fixed with either formaldehyde (F panels) or methanol-acetone (M panels) and labeled with antibody 1 (A to C) or antibody 2 (D) against Nup50 for visualization by indirect immunofluorescence microscopy.
FIG. 4
Distribution of Nup50 by subcellular fractionation. (A) Isolated rat liver nuclei (Nuc) were digested with DNase-RNase to release intranuclear contents from the NE and were centrifuged at low speed to yield a supernatant (Sup) and pellet (Pel). (B) Isolated rat liver NE, derived by nuclease-salt extraction of nuclei, were suspended in a solution containing 0.5 M NaCl and were centrifuged to yield a supernatant and a pellet. (C) NRK cells (Cell) were permeabilized, digested with DNase-RNase, suspended in buffer containing 0.5 M NaCl, and centrifuged to yield a supernatant and pellet. (D) Rat liver NEs were solubilized in nonionic detergent buffer and were passed over a WGA affinity column, yielding bound and unbound fractions. Samples of the various subcellular fractions were electrophoresed on SDS gels and analyzed by immunoblotting with antibody 1 or the monoclonal antibody RL1 as indicated.
FIG. 5
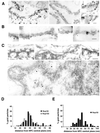
Immunoelectron microscopic localization of Nup50 in NEs and NRK cells. (A) Isolated rat liver NEs were labeled by indirect immunogold techniques with anti-Nup50 antibodies (antibody 1). Shown are cross-sectional (left two panels) and tangential (right panels) views of the NE depicting the labeling pattern that was obtained. Undigested chromatin associated with the inner nuclear membrane (Ch) allows distinction between the cytoplasmic and nucleoplasmic sides of the NPC. Arrowheads denote NPCs that are labeled with anti-Nup50 antibodies. (B) Isolated rat liver NEs were processed by indirect immunogold labeling to simultaneously localize Nup50 (antibody 1, 10-nm gold, arrow in left panel) and Nup153 (RL11, 5-nm gold, arrowhead in left panel). (C) NRK cells were permeabilized by freeze-thawing, and Nup50 was localized by indirect immunogold labeling. The top row of panels depicts cross-sectional views of the NE, which reveal gold labeling exclusively on the nucleoplasmic side of the NPC. The bottom panel presents a cross-section through the nucleus, showing that gold labeling is present in more internal nuclear regions (arrowheads), as well as at the nucleoplasmic surface of the NPC. Bars, 100 nm. (D) Quantification of the localization of Nup50 and Nup153 in isolated rat liver NEs with respect to the NPC midplane. One hundred eighteen gold particles were counted for Nup50, and 110 were counted for Nup153. (E) Quantification of the localization of Nup50 at the NE of NRK cells with respect to the NPC midplane. Eighty-four gold particles were counted.
FIG. 6
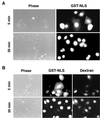
Analysis of nuclear protein export in NRK cells injected with anti-Nup50 antibodies. The nuclei of cells were injected with a marker 100-kDa fluorescent dextran, together with GST-NES mixed with buffer (A) or with GST-NES mixed with antibody 1 (B), antibody 2 (C), or antibody 1 plus immunizing antigen (D), as shown. After 30 min at 37°C, cells were fixed, and the localization of GST-NES was determined by indirect immunofluorescence microscopy. Injected nuclei are denoted by the fluorescent dextran.
FIG. 7
Analysis of nuclear protein import in NRK cells injected with anti-Nup50 antibodies. (A) Cells were injected in the cytoplasm with GST-NLS. After either 5 or 30 min at 37°C, cells were fixed and the GST-NLS was localized by indirect immunofluorescence microscopy. (B) Cells were injected into the nucleus with a mixture of a marker (100-kDa fluorescent dextran) and antibody 1. They were then injected into the cytoplasm with GST-NLS. After either 5 or 30 min at 37°C, cells were fixed and the GST-NLS was localized by indirect immunofluorescence microscopy. Injected nuclei are denoted by the fluorescent dextran.
FIG. 8
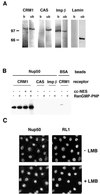
Interaction of nuclear transport receptors with Nup50. (A) NRK cells were solubilized in NP-40 buffer, and extracts were immunoprecipitated with anti-Nup50 antibodies (antibody 1). Material bound to the antibody beads (b) and the unbound material (ub) were electrophoresed on an SDS gel and analyzed by immunoblotting with anti-CRM1, anti-CAS, anti-importin β, or anti-lamin antibodies. Note that the sample loaded in the bound lanes represents sevenfold more cell equivalents than the material in the unbound lanes. (B) Sepharose beads coupled with a Nup50 fragment (Nup50) or with BSA were incubated with recombinant CRM1, CAS, or importin β in the absence or presence of RanGMP-PNP or cytochrome c coupled with NES peptides (cc-NES) as indicated. Samples were analyzed by immunoblotting with antibodies recognizing the His6 tag of the recombinant receptors. An equivalent amount of the bound material was loaded in all cases. Moreover, the input material contained nearly equal quantities of all transport receptors, as determined by protein analysis. (C) NRK cells were incubated at 37°C for 4 h in growth medium containing 100 nM leptomycin B (+LMB) or lacking leptomycin B (−LMB). They were then fixed with methanol-acetone (see Materials and Methods) and examined by confocal microscopy after double immunofluorescence staining with anti-Nup50 (antibody 1) or RL1, as indicated.
Similar articles
- Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex.
Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Smitherman M, et al. Mol Cell Biol. 2000 Aug;20(15):5631-42. doi: 10.1128/MCB.20.15.5631-5642.2000. Mol Cell Biol. 2000. PMID: 10891500 Free PMC article. - Nup50/Npap60 function in nuclear protein import complex disassembly and importin recycling.
Matsuura Y, Stewart M. Matsuura Y, et al. EMBO J. 2005 Nov 2;24(21):3681-9. doi: 10.1038/sj.emboj.7600843. Epub 2005 Oct 13. EMBO J. 2005. PMID: 16222336 Free PMC article. - Separate nuclear import pathways converge on the nucleoporin Nup153 and can be dissected with dominant-negative inhibitors.
Shah S, Forbes DJ. Shah S, et al. Curr Biol. 1998 Dec 17-31;8(25):1376-86. doi: 10.1016/s0960-9822(98)00018-9. Curr Biol. 1998. PMID: 9889100 - Npap60: a new player in nuclear protein import.
Moore MS. Moore MS. Trends Cell Biol. 2003 Feb;13(2):61-4. doi: 10.1016/s0962-8924(02)00044-2. Trends Cell Biol. 2003. PMID: 12559755 Review. - Distinct nuclear import and export pathways mediated by members of the karyopherin beta family.
Moroianu J. Moroianu J. J Cell Biochem. 1998 Aug 1;70(2):231-9. J Cell Biochem. 1998. PMID: 9671229 Review.
Cited by
- Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions.
Raices M, D'Angelo MA. Raices M, et al. Nat Rev Mol Cell Biol. 2012 Nov;13(11):687-99. doi: 10.1038/nrm3461. Nat Rev Mol Cell Biol. 2012. PMID: 23090414 Review. - The importin β binding domain as a master regulator of nucleocytoplasmic transport.
Lott K, Cingolani G. Lott K, et al. Biochim Biophys Acta. 2011 Sep;1813(9):1578-92. doi: 10.1016/j.bbamcr.2010.10.012. Epub 2010 Oct 26. Biochim Biophys Acta. 2011. PMID: 21029753 Free PMC article. Review. - Evolutionary divergence of the nuclear pore complex from fungi to metazoans.
Chopra K, Bawaria S, Chauhan R. Chopra K, et al. Protein Sci. 2019 Mar;28(3):571-586. doi: 10.1002/pro.3558. Epub 2018 Dec 24. Protein Sci. 2019. PMID: 30488506 Free PMC article. - Nucleoporin Nup50 stabilizes closed conformation of armadillo repeat 10 in importin α5.
Pumroy RA, Nardozzi JD, Hart DJ, Root MJ, Cingolani G. Pumroy RA, et al. J Biol Chem. 2012 Jan 13;287(3):2022-31. doi: 10.1074/jbc.M111.315838. Epub 2011 Nov 30. J Biol Chem. 2012. PMID: 22130666 Free PMC article. - The Nup153-Nup50 protein interface and its role in nuclear import.
Makise M, Mackay DR, Elgort S, Shankaran SS, Adam SA, Ullman KS. Makise M, et al. J Biol Chem. 2012 Nov 9;287(46):38515-22. doi: 10.1074/jbc.M112.378893. Epub 2012 Sep 24. J Biol Chem. 2012. PMID: 23007389 Free PMC article.
References
- Adam S A. Transport pathways of macromolecules between the nucleus and the cytoplasm. Curr Opin Cell Biol. 1999;11:402–406. - PubMed
- Arts G J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM019085/GM/NIGMS NIH HHS/United States
- R37 GM036745/GM/NIGMS NIH HHS/United States
- GM36745/GM/NIGMS NIH HHS/United States
- R01 GM036745/GM/NIGMS NIH HHS/United States
- F32 GM19085/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases