Identification of the cytolinker plectin as a major early in vivo substrate for caspase 8 during CD95- and tumor necrosis factor receptor-mediated apoptosis - PubMed (original) (raw)
Identification of the cytolinker plectin as a major early in vivo substrate for caspase 8 during CD95- and tumor necrosis factor receptor-mediated apoptosis
A H Stegh et al. Mol Cell Biol. 2000 Aug.
Abstract
Caspase 8 plays an essential role in the execution of death receptor-mediated apoptosis. To determine the localization of endogenous caspase 8, we used a panel of subunit-specific anti-caspase 8 monoclonal antibodies in confocal immunofluorescence microscopy. In the human breast carcinoma cell line MCF7, caspase 8 predominantly colocalized with and bound to mitochondria. After induction of apoptosis through CD95 or tumor necrosis factor receptor I, active caspase 8 translocated to plectin, a major cross-linking protein of the three main cytoplasmic filament systems, whereas the caspase 8 prodomain remained bound to mitochondria. Plectin was quantitatively cleaved by caspase 8 at Asp 2395 in the center of the molecule in all cells tested. Cleavage of plectin clearly preceded that of other caspase substrates such as poly(ADP-ribose) polymerase, gelsolin, cytokeratins, or lamin B. In primary fibroblasts from plectin-deficient mice, apoptosis-induced reorganization of the actin cytoskeleton, as seen in wild-type cells, was severely impaired, suggesting that during apoptosis, plectin is required for the reorganization of the microfilament system.
Figures
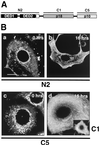
FIG. 1
Localization of caspase 8 cleavage products in MCF7-Fas cells during CD95-mediated apoptosis. (A) Binding specificities of the anti-caspase 8 MAbs. N2 recognizes the caspase 8 prodomain containing the two DEDs. C1 and C5 are directed against the active subunits of caspase 8, p18 and p10, respectively. (B) MCF7-Fas cells were left untreated (0 h) or treated with anti-CD95 (2 μg/ml) for 16 h and subjected to laser scanning immunofluorescence microscopy using anti-caspase 8 MAbs C5, C1, and N2, all directly labeled with FITC. Bar, 10 μm. The specificity of the procaspase 8 staining was confirmed by using unlabeled primary antibodies and FITC-coupled secondary antibodies. These indirect immunofluorescence stainings gave results similar to those obtained by the direct immunofluorescence (data not shown). In addition, stainings of caspase 8-deficient Jurkat cells (provided by J. Blenis, Boston, Mass.) (25) with the anti-caspase 8 antibody C5 were negative (data not shown). Furthermore, direct immunofluorescence with an isotype-matched FITC-labeled control antibody resulted in only background staining (data not shown).
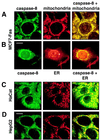
FIG. 2
Caspase 8 colocalizes with and binds to mitochondria. (A and B) The breast carcinoma cell line MCF7-Fas was double stained for caspase 8 (anti-caspase 8 C5, FITC labeled; left column) and mitochondria (antimitochondrial antigen, second antibody, PE labeled; center column) (A) or for caspase 8 and ER (anti-PDI antibody, second antibody, PE labeled) (B). The right column presents the overlay of the two stainings. (C and D) HaCat keratinocytes (C) and HepG2 hepatocarcinoma cells (D) were double stained for caspase 8 and mitochondria as for panel A. Bar, 10 μm. (E and F) Biochemical analysis of the subcellular localization of caspase 8. (E) In vitro-translated [35S]caspase-8/a (CASP-8/a) and [35S]FADD were incubated with purified mitochondria, and the amount of bound (B) and unbound (U) in vitro-translated material was determined by SDS-PAGE (12% gel) and subsequent autoradiography. The migration positions of caspase 8 and FADD are indicated. (F) Subcellular fractionation of MCF7-Fas cells. MCF7-Fas cells (5 × 107) were subjected to subcellular fractionation into mitochondria and cytoplasm after treatment with anti-CD95. The Western blot was developed with anti-caspase 8 MAb C15. The migration positions of caspase 8/a and 8/b and the caspase 8 active subunit p18 are indicated. To assess the purity of the mitochondrial (M) and cytoplasmic (C) fractions, a Western blot was developed with antibodies directed against mitochondrial marker proteins p60 (recognized by the antimitochondrial antibody also used for immunofluorescence), cytochrome c (cyt c), and as a cytoplasmic marker FADD.
FIG. 2
Caspase 8 colocalizes with and binds to mitochondria. (A and B) The breast carcinoma cell line MCF7-Fas was double stained for caspase 8 (anti-caspase 8 C5, FITC labeled; left column) and mitochondria (antimitochondrial antigen, second antibody, PE labeled; center column) (A) or for caspase 8 and ER (anti-PDI antibody, second antibody, PE labeled) (B). The right column presents the overlay of the two stainings. (C and D) HaCat keratinocytes (C) and HepG2 hepatocarcinoma cells (D) were double stained for caspase 8 and mitochondria as for panel A. Bar, 10 μm. (E and F) Biochemical analysis of the subcellular localization of caspase 8. (E) In vitro-translated [35S]caspase-8/a (CASP-8/a) and [35S]FADD were incubated with purified mitochondria, and the amount of bound (B) and unbound (U) in vitro-translated material was determined by SDS-PAGE (12% gel) and subsequent autoradiography. The migration positions of caspase 8 and FADD are indicated. (F) Subcellular fractionation of MCF7-Fas cells. MCF7-Fas cells (5 × 107) were subjected to subcellular fractionation into mitochondria and cytoplasm after treatment with anti-CD95. The Western blot was developed with anti-caspase 8 MAb C15. The migration positions of caspase 8/a and 8/b and the caspase 8 active subunit p18 are indicated. To assess the purity of the mitochondrial (M) and cytoplasmic (C) fractions, a Western blot was developed with antibodies directed against mitochondrial marker proteins p60 (recognized by the antimitochondrial antibody also used for immunofluorescence), cytochrome c (cyt c), and as a cytoplasmic marker FADD.
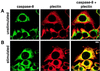
FIG. 3
Colocalization of caspase 8 with plectin during CD95-mediated apoptosis. Untreated (A) and anti-CD95-treated (2 μg/ml anti-CD95, 16 h) (B) were double stained for caspase 8 (left column) and plectin (center column). The right column presents the overlay of the two stainings. Panels show representative midsections through the cells. Bar, 10 μm.
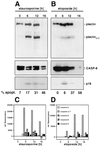
FIG. 4
Cleavage of plectin during death receptor-mediated apoptosis. (A) MCF7-Fas cells were treated with anti-CD95 (2 μg/ml) for the indicated time periods. Plectin was enriched as described in Materials and Methods. For caspase 8 detection, Triton X-100 cell lysates were prepared (see Materials and Methods). Plectin and caspase 8 cleavage was followed by Western blotting using MAb C15 and the anti-plectin-C antibody. For inhibition of caspase 8 activation, cells were incubated for 30 min with 20 μM zVAD-fmk prior to addition of anti-CD95 (2 μg/ml). Migration positions of plectin, caspase 8 (CASP-8), and the caspase 8 active subunit p18 are shown. Apoptosis was quantified using CELLocate coverslips (see Materials and Methods). (B) As for panel A except that cells were treated with TNF-α (20 ng/ml) and CHX (1 μg/ml). (C and D) Caspase activities in cellular lysates of cells treated with anti-CD95 (2 μg/ml) or TNF-α (20 ng/ml) and CHX (1 μg/ml) as in panel B were determined as described in Materials and Methods. Note that in addition to early activation of caspase 8 during CD95-mediated apoptosis (C), a late activation of caspase 7 was detectable, consistent with an earlier report (58).
FIG. 5
Cleavage of plectin during drug-induced apoptosis. Jurkat cells were incubated with staurosporine (1 μM) (A) or etoposide (20 μg/ml) (B) for the indicated time periods. Analysis of plectin and caspase 8 cleavage fragments was done as described for Fig. 4A and B; quantification of the apoptotic cells was done as described in Materials and Methods. (C and D) Caspase activities during drug-induced apoptosis, determined as described in the legend for Fig. 4C and D.
FIG. 6
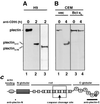
Plectin is cleaved by caspase 8 in lymphoid cells. (A) The T-lymphoma cell line H9 was treated with anti-CD95 (2 μg/ml) for the indicated periods of time. The Western blot was developed with anti-plectin-C (lanes 1 and 2) and anti-plectin-N (lane 3) antisera. Migration positions of plectin, of plectincl-C, and of plectincl-N are indicated. (B) The T-lymphoma cell line CEM transfected with vector (vec) or Bcl-xL was treated with anti-CD95 (2 μg/ml) for the indicated times, and the Western blotting for plectin was performed as for panel A. (C) Locations of the putative caspase cleavage site and the antiplectin antiserum recognition sites within the plectin molecule. The box indicates the region of the recombinant plectin fragment used in experiments shown in Fig. 7C to E.
FIG. 7
Plectin is directly cleaved by caspase 8 in the center of the molecule. (A) Active DISC prepared from SKW6.4 cells as described in Materials and Methods was added to an IF preparation obtained from untreated MCF7-Fas cells, incubated overnight at 4°C, and immunoblotted with the anti-plectin-C antiserum. Migration positions of full-length plectin and plectincl-C are indicated. (B) In vitro cleavage of plectin by recombinant caspases. IF preparations obtained from untreated MCF7-Fas cells were incubated with recombinant caspases (active concentration, 10 μM each) for 8 h, 12 h, and 36 h. The Western blot was developed with the anti-plectin C antiserum. Migration positions of plectin and plectincl-C were identical to those in Fig. 5A and B, lanes 2. The densitometric analysis of this Western blot is shown as ratio plectincl/plectin. Data points represent control (▴), caspase 3 (○), caspase 6 (□), caspase 7 (■), caspase 8 (●), and caspase 10 (◊). (C) In vitro cleavage of a recombinant plectin fragment encompassing the putative cleavage site for caspase 8 with recombinant caspases 3, 6, 7, 8, and 10 (active concentration, 40 μM each) or with cleavage buffer (lane C). Proteins were analyzed by SDS-PAGE and Coomassie brilliant blue staining. Edman degradation showed that the 22-kDa fragment generated by caspase 8 represents the N-terminal half of the plectin fragment. The C-terminal half was not found likely due to secondary proteolytic degradation. (D) Schematic representation of the rat plectin fragment covering the part of the plectin rod containing potential caspase cleavage sites. Amino acid positions of the aspartic acid residues that were replaced by alanine are indicated. (E) In vitro cleavage of recombinant plectin fragment mutants. Mutant proteins were incubated in the absence (−) or presence (+) of active caspase 8 for 24 h and analyzed as for panel C. Migration positions of the full-length protein (filled arrowheads) and of the cleavage fragment (open arrowheads) are indicated in panels C and E.
FIG. 8
Cleavage of plectin precedes cleavage of CK18, lamin B, and gelsolin during CD95-mediated apoptosis. (A) MCF7-Fas cells were stimulated with anti-CD95 (2 μg/ml) for the indicated time periods or treated with 20 μM zVAD-fmk prior to addition of 2 μg of anti-CD95 per ml. The corresponding IF preparations were analyzed by Western blotting using the anti-plectin-C antiserum. (B to D) Cells were treated as for panel A, but Triton X-100 cell lysates were prepared (see Materials and Methods). Western blots were developed with anti-CK 8 (B), anti-CK 18 (C), or anti-lamin B (D) antibody. (E to F) Jurkat T cells were stimulated with anti-CD95 (2 μg/ml) for the indicated time periods. Plectin IF preparations (E) or Triton X-100 cell lysates (F) were analyzed for plectin or gelsolin. Migration positions of the full-length proteins are indicated by filled arrowheads; migration positions of the cleavage products are marked by open arrowheads. Molecular masses of the cleavage products: plectin, 200 kDa; CK18, 28 kDa; lamin B, 28 and 21 kDa; gelsolin, 48 kDa.
FIG. 9
Plectin is required for actin reorganization during CD95-mediated apoptosis. (A) Staining of primary fibroblasts from wild-type (wt) and plectin-deficient (knockout [k.o.]) mice with the anti-plectin-C antiserum using immunofluorescence microscopy. (B) Sensitivity of IFN-γ-treated primary fibroblasts to CD95-mediated DNA fragmentation. Cells were treated with IFN-γ for 36 h, and apoptosis was induced by using anti-CD95 antibody Jo2 (10 μg/ml; Pharmingen), protein A (10 ng/ml), and CHX (1 μg/ml). (C) Staining of actin in primary fibroblasts after incubation with anti-CD95 for 0 or 12 h. Arrowheads point to membrane ruffles.
FIG. 10
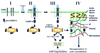
Model of apoptosis signaling in MCF7-Fas cells. MCF7-Fas cells express CD95 on the cell surface. Most procaspase 8 in these cells is localized to mitochondria (I). Upon CD95 triggering, procaspase 8 (pro-CASP-8) is recruited to the DISC and subsequently activated by proteolytical cleavage, resulting in release of active subunits (orange and purple boxes) into the cytoplasm (II) (39). Recent data indicate that active caspase 8 directly and specifically activates procaspase 8 on mitochondria (III) (unpublished data). In contrast to the prodomain (green box) that remains bound at the mitochondria, the active caspase 8 subunits translocate to plectin and cleave this cytoskeleton-associated regulatory protein (IV). This cleavage may be required for the reorganization of the actin cytoskeleton typical for apoptosis by an unknown mechanism. The DISC is shown in a simplified form without FADD. Cyt c, cytochrome c.
Similar articles
- NuMA and nuclear lamins behave differently in Fas-mediated apoptosis.
Taimen P, Kallajoki M. Taimen P, et al. J Cell Sci. 2003 Feb 1;116(Pt 3):571-83. doi: 10.1242/jcs.00227. J Cell Sci. 2003. PMID: 12508117 - Caspase 8-mediated cleavage of plectin precedes F-actin breakdown in acinar cells during pancreatitis.
Beil M, Leser J, Lutz MP, Gukovskaya A, Seufferlein T, Lynch G, Pandol SJ, Adler G. Beil M, et al. Am J Physiol Gastrointest Liver Physiol. 2002 Mar;282(3):G450-60. doi: 10.1152/ajpgi.00042.2001. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 11841995 - alpha-fetoprotein causes apoptosis in tumor cells via a pathway independent of CD95, TNFR1 and TNFR2 through activation of caspase-3-like proteases.
Dudich E, Semenkova L, Dudich I, Gorbatova E, Tochtamisheva N, Tatulov E, Nikolaeva M, Sukhikh G. Dudich E, et al. Eur J Biochem. 1999 Dec;266(3):750-61. doi: 10.1046/j.1432-1327.1999.00868.x. Eur J Biochem. 1999. PMID: 10583368 - Epoxycyclohexenone inhibits Fas-mediated apoptosis by blocking activation of pro-caspase-8 in the death-inducing signaling complex.
Miyake Y, Kakeya H, Kataoka T, Osada H. Miyake Y, et al. J Biol Chem. 2003 Mar 28;278(13):11213-20. doi: 10.1074/jbc.M209610200. Epub 2003 Jan 24. J Biol Chem. 2003. PMID: 12551927
Cited by
- DEDD regulates degradation of intermediate filaments during apoptosis.
Lee JC, Schickling O, Stegh AH, Oshima RG, Dinsdale D, Cohen GM, Peter ME. Lee JC, et al. J Cell Biol. 2002 Sep 16;158(6):1051-66. doi: 10.1083/jcb.200112124. Epub 2002 Sep 16. J Cell Biol. 2002. PMID: 12235123 Free PMC article. - Plectin deficiency on cytoskeletal disorganization and transformation of human liver cells in vitro.
Liu YH, Cheng CC, Ho CC, Chao WT, Pei RJ, Hsu YH, Ho LC, Shiu BH, Lai YS. Liu YH, et al. Med Mol Morphol. 2011 Mar;44(1):21-6. doi: 10.1007/s00795-010-0499-y. Med Mol Morphol. 2011. PMID: 21424933 - Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve.
Castañón MJ, Walko G, Winter L, Wiche G. Castañón MJ, et al. Histochem Cell Biol. 2013 Jul;140(1):33-53. doi: 10.1007/s00418-013-1102-0. Epub 2013 Jun 9. Histochem Cell Biol. 2013. PMID: 23748243 Free PMC article. Review. - Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation.
Gilbert S, Loranger A, Daigle N, Marceau N. Gilbert S, et al. J Cell Biol. 2001 Aug 20;154(4):763-73. doi: 10.1083/jcb.200102130. J Cell Biol. 2001. PMID: 11514590 Free PMC article.
References
- Boesen-de Cock J G, Tepper A D, de Vries E, van Blitterswijk W J, Borst J. Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem. 1999;274:14255–14261. - PubMed
- Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. - PubMed
- Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous