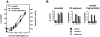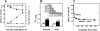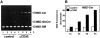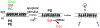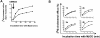Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology - PubMed (original) (raw)
Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology
A D Tepper et al. J Cell Biol. 2000.
Abstract
Apoptosis is generally accompanied by a late phase of ceramide (Cer) production, the significance of which is unknown. This study describes a previously unrecognized link between Cer accumulation and phosphatidylserine (PS) exposure at the cell surface, a characteristic of the execution phase of apoptosis resulting from a loss of plasma membrane phospholipid asymmetry. Using a fluorescent sphingomyelin (SM) analogue, N-(N-[6-[(7-nitrobenz-2-oxa-1, 3-diazol-4-yl)amino]caproyl]-sphingosylphosphorylcholine (C(6)-NBD-SM), we show that Cer is derived from SM, initially located in the outer leaflet of the plasma membrane, which gains access to a cytosolic SMase by flipping to the inner leaflet in a process of lipid scrambling paralleling PS externalization. Lipid scrambling is both necessary and sufficient for SM conversion: Ca(2+) ionophore induces both PS exposure and SM hydrolysis, whereas scrambling-deficient Raji cells do not show PS exposure or Cer formation. Cer is not required for mitochondrial or nuclear apoptotic features since these are still observed in Raji cells. SM hydrolysis facilitates cholesterol efflux to methyl-beta-cyclodextrin, which is indicative of a loss of tight SM-cholesterol interaction in the plasma membrane. We provide evidence that these biophysical alterations in the lipid bilayer are essential for apoptotic membrane blebbing/vesiculation at the cell surface: Raji cells show aberrant apoptotic morphology, whereas replenishment of hydrolyzed SM by C(6)- NBD-SM inhibits blebbing in Jurkat cells. Thus, SM hydrolysis, during the execution phase of apoptosis, results from a loss of phospholipid asymmetry and contributes to structural changes at the plasma membrane.
Figures
Figure 1
Cer formation is associated with PS exposure. (A) Time course of CD95-induced Cer formation, PS exposure, and nuclear fragmentation in Jurkat cells. Sphingolipids were labeled to equilibrium with [14C]serine. Cells were stimulated with anti-CD95 mAb (200 ng/ml) for the indicated periods of time. Cer formation was assessed by TLC. PS exposure and nuclear fragmentation were analyzed by annexinV-FITC staining of intact cells or propidium iodide staining of nuclei, respectively. Data shown are of one experiment performed three times with similar results. Cer data points represent the mean ± SD of three individual measurements. The other data points represent individual measurements. (B) CD95- and etoposide-induced Cer formation, PS exposure, and nuclear fragmentation were determined in parallel in the presence and absence of caspase inhibitors. Jurkat cells were radiolabeled as described above, left untreated (black bars) or preincubated for 2 h with zVAD-fmk (50 μM; gray bars) or DEVD-CHO (100 μM; white bars), and then exposed to anti-CD95 mAb (200 ng/ml) during 4 h or etoposide (10 μg/ml) during 16 h. Data are individual measurements within one experiment representative of at least four separate experiments.
Figure 2
CD95-induced Cer is derived from SM, not from de novo biosynthesis. (A) Jurkat cells labeled with [14C]serine were stimulated with anti-CD95 mAb (200 ng/ml; closed symbols) for indicated time periods or left untreated (open symbols), after which lipids were extracted and analyzed by TLC. Data are expressed as a percentage of SM (circles) and multiple-fold increase in Cer (triangles) relative to an untreated control sample harvested just before the start of the experiment. (B) Effect of fumonisin B1 (FB1) on CD95-induced Cer formation. Jurkat cells labeled to equilibrium with [14C]serine were preincubated for 24 h without (medium) or with 25 μM FB1 (+FB1), washed, resuspended in medium with or without 25 μM FB1, and stimulated with anti-CD95 mAb (200 ng/ml; closed bars) or left untreated (open bars). After 3 h, lipids were extracted and Cer was quantified. Data (means ± SD) represent two independent experiments. The Cer response is indicated as a percentage of untreated control cells. Note that, as expected, FB1 reduces absolute [14C]Cer levels as defined in Materials and Methods. (Inset) Effect of FB1 on SM synthesis. Cells were preincubated with 25 μM FB1 for time periods indicated, after which [14C]serine was added for an additional 36 h. Lipids were extracted and SM was quantified. Results are expressed as a percentage of untreated control cells and are representative of two experiments. (C) The pool of SM hydrolyzed upon CD95 stimulation is fully accessible to an exogenous SMase. Jurkat cells metabolically labeled with [14C]choline were treated with B. cereus SMase (200 mU/ml) alone (closed circles) or with SMase plus anti-CD95 mAb (200 ng/ml; open squares). At various time points, samples were analyzed for radiolabeled SM and PC content. The ratio of SM relative to PC is expressed as a percentage of control. This experiment was performed twice with similar results.
Figure 3
Cer is derived from the SM originally present in the outer leaflet of the plasma membrane. (A) CD95 induces hydrolysis of exogenous fluorescent NBD-SM. Jurkat cells were incubated with 4 μM NBD-SM 10 min before CD95 stimulation (200 ng mAb/ml) for the times indicated. NBD-lipids were extracted, separated by TLC, visualized under UV light, and photographed. The identities of NBD-Cer and NBD-GlcCer (NBD-glucosylceramide) were established using co-chromatography of commercially obtained lipid standards. NBD-Cer formation was most easily detected when the lipid probe was continuously present during stimulation, but significant NBD-SM hydrolysis in CD95-stimulated cells was also observed when NBD-SM was added during the last 15 min of incubation (results not shown). (B) Quantitation of the NBD-Cer spots shown in A by fluorescence spectrophotometry (expression in arbitrary units). (C) NBD-Cer is derived from outer leaflet NBD-SM. Jurkat cells were incubated with 4 μM NBD-SM during 30 min at 37°C to allow labeling of intracellular and plasma membranes, followed or not followed by BSA back extraction (see Materials and Methods). Cells resuspended in H/H were warmed to 37°C and exposed to anti-CD95 mAb (200 ng/ml; +) or left untreated (−). Lipids were extracted and analyzed by TLC after the indicated time periods. The BSA extraction protocol did not affect apoptosis induction, as verified by nuclear fragmentation (data not shown). (D) DNA damage–induced NBD-SM hydrolysis in SKW6.4 cells, 14 or 16 h after exposure to etoposide (5 μg/ml; Eto) or radiation (20 Gy; γ-IR), NBD-SM (4 μM) was presented during 20 min to SKW6.4. TLC analysis indicates increased NBD-SM hydrolysis to NBD-Cer in cells undergoing apoptosis relative to untreated control cells (con).
Figure 3
Cer is derived from the SM originally present in the outer leaflet of the plasma membrane. (A) CD95 induces hydrolysis of exogenous fluorescent NBD-SM. Jurkat cells were incubated with 4 μM NBD-SM 10 min before CD95 stimulation (200 ng mAb/ml) for the times indicated. NBD-lipids were extracted, separated by TLC, visualized under UV light, and photographed. The identities of NBD-Cer and NBD-GlcCer (NBD-glucosylceramide) were established using co-chromatography of commercially obtained lipid standards. NBD-Cer formation was most easily detected when the lipid probe was continuously present during stimulation, but significant NBD-SM hydrolysis in CD95-stimulated cells was also observed when NBD-SM was added during the last 15 min of incubation (results not shown). (B) Quantitation of the NBD-Cer spots shown in A by fluorescence spectrophotometry (expression in arbitrary units). (C) NBD-Cer is derived from outer leaflet NBD-SM. Jurkat cells were incubated with 4 μM NBD-SM during 30 min at 37°C to allow labeling of intracellular and plasma membranes, followed or not followed by BSA back extraction (see Materials and Methods). Cells resuspended in H/H were warmed to 37°C and exposed to anti-CD95 mAb (200 ng/ml; +) or left untreated (−). Lipids were extracted and analyzed by TLC after the indicated time periods. The BSA extraction protocol did not affect apoptosis induction, as verified by nuclear fragmentation (data not shown). (D) DNA damage–induced NBD-SM hydrolysis in SKW6.4 cells, 14 or 16 h after exposure to etoposide (5 μg/ml; Eto) or radiation (20 Gy; γ-IR), NBD-SM (4 μM) was presented during 20 min to SKW6.4. TLC analysis indicates increased NBD-SM hydrolysis to NBD-Cer in cells undergoing apoptosis relative to untreated control cells (con).
Figure 4
Scrambling of plasma membrane phospholipids, monitored by annexin V staining (PS exposure), is necessary and sufficient for Cer formation. (A) Raji cells show no CD95-induced PS exposure, nor Cer formation, yet exhibit loss of mitochondrial transmembrane potential (ΔΨm) and nuclear fragmentation. Cells labeled with [14C]serine were exposed to anti-CD95 mAb (200 ng/ml) for the indicated time periods and [14C]Cer was quantified. PS exposure (annexin V staining), ΔΨm (measured with the fluorescent dye DiOC6(3)), and nuclear fragmentation were determined in parallel. (B) Absence of NBD-SM hydrolysis to NBD-Cer in CD95-stimulated Raji cells. Cells were incubated with 4 μM NBD-SM 10 min before the addition of anti-CD95 mAb (200 ng/ml). At the indicated time points, total lipids were extracted and separated by TLC. A commercial NBD-Cer standard was run in parallel. (C) Ionomycin induces PS exposure in Jurkat but not in Raji cells. Cells were exposed to 5 μM ionomycin for the indicated time periods or left untreated (control; con), and PS exposure was detected using FITC-labeled annexin V. Histograms are representative of at least three experiments. (D) Ionomycin induces NBD-SM hydrolysis to NBD-Cer in Jurkat but not in Raji cells. Cells preincubated for 10 min with 4 μM NBD-SM were exposed to 5 μM ionomycin or its solvent (control) for the times indicated, after which lipids were extracted and separated by TLC. NBD-Cer fluorescence was quantified using fluorescence spectrophotometry.
Figure 4
Scrambling of plasma membrane phospholipids, monitored by annexin V staining (PS exposure), is necessary and sufficient for Cer formation. (A) Raji cells show no CD95-induced PS exposure, nor Cer formation, yet exhibit loss of mitochondrial transmembrane potential (ΔΨm) and nuclear fragmentation. Cells labeled with [14C]serine were exposed to anti-CD95 mAb (200 ng/ml) for the indicated time periods and [14C]Cer was quantified. PS exposure (annexin V staining), ΔΨm (measured with the fluorescent dye DiOC6(3)), and nuclear fragmentation were determined in parallel. (B) Absence of NBD-SM hydrolysis to NBD-Cer in CD95-stimulated Raji cells. Cells were incubated with 4 μM NBD-SM 10 min before the addition of anti-CD95 mAb (200 ng/ml). At the indicated time points, total lipids were extracted and separated by TLC. A commercial NBD-Cer standard was run in parallel. (C) Ionomycin induces PS exposure in Jurkat but not in Raji cells. Cells were exposed to 5 μM ionomycin for the indicated time periods or left untreated (control; con), and PS exposure was detected using FITC-labeled annexin V. Histograms are representative of at least three experiments. (D) Ionomycin induces NBD-SM hydrolysis to NBD-Cer in Jurkat but not in Raji cells. Cells preincubated for 10 min with 4 μM NBD-SM were exposed to 5 μM ionomycin or its solvent (control) for the times indicated, after which lipids were extracted and separated by TLC. NBD-Cer fluorescence was quantified using fluorescence spectrophotometry.
Figure 4
Scrambling of plasma membrane phospholipids, monitored by annexin V staining (PS exposure), is necessary and sufficient for Cer formation. (A) Raji cells show no CD95-induced PS exposure, nor Cer formation, yet exhibit loss of mitochondrial transmembrane potential (ΔΨm) and nuclear fragmentation. Cells labeled with [14C]serine were exposed to anti-CD95 mAb (200 ng/ml) for the indicated time periods and [14C]Cer was quantified. PS exposure (annexin V staining), ΔΨm (measured with the fluorescent dye DiOC6(3)), and nuclear fragmentation were determined in parallel. (B) Absence of NBD-SM hydrolysis to NBD-Cer in CD95-stimulated Raji cells. Cells were incubated with 4 μM NBD-SM 10 min before the addition of anti-CD95 mAb (200 ng/ml). At the indicated time points, total lipids were extracted and separated by TLC. A commercial NBD-Cer standard was run in parallel. (C) Ionomycin induces PS exposure in Jurkat but not in Raji cells. Cells were exposed to 5 μM ionomycin for the indicated time periods or left untreated (control; con), and PS exposure was detected using FITC-labeled annexin V. Histograms are representative of at least three experiments. (D) Ionomycin induces NBD-SM hydrolysis to NBD-Cer in Jurkat but not in Raji cells. Cells preincubated for 10 min with 4 μM NBD-SM were exposed to 5 μM ionomycin or its solvent (control) for the times indicated, after which lipids were extracted and separated by TLC. NBD-Cer fluorescence was quantified using fluorescence spectrophotometry.
Figure 5
Plasma membrane SM content determines surface morphology of apoptotic cells. Raji and Jurkat cells suspended at 2.5 × 106 cells per ml in H/H medium without (medium) or with 4 μM NBD-SM (NBD-SM) were left untreated (control) or exposed to anti-CD95 mAb (200 ng/ml) for 6 h (Raji) or 1.5 h (Jurkat). Cells were viewed and photographed using a light transmission microscope with a 40× objective.
Figure 7
Schematic representation of the proposed mechanism and relevance of Cer formation during the execution phase of apoptosis. In viable cells, SM (red) and PC (blue) localize to the exoplasmic leaflet of the plasma membrane, while PS and PE (white) are sequestered in the inner leaflet. Cholesterol (Chol; green) partitions between both leaflets, but only the preferential clustering with outer leaflet SM is indicated, for reasons of clarity. An apoptotic stimulus or elevated calcium induces loss of the asymmetric phospholipid distribution, and SM appears in the inner leaflet, where it is immediately hydrolyzed to Cer by an intracellular neutral sphingomyelinase (nSMase). Hydrolysis of SM disturbs its tight interaction with Chol, resulting in redistribution of Chol from the plasma membrane towards the cell interior or an extracellular acceptor. Reduced SM and Chol content alters biophysical properties of the lipid bilayer, allowing morphological changes such as membrane blebbing and vesicle shedding.
Figure 6
Enhanced efflux of [3H]cholesterol from the plasma membrane during apoptosis induction. (A) Depletion of SM from the plasma membrane by exogenous SMase enhances efflux of membrane cholesterol to methyl-β-cyclodextrin (MβCD), which is a specific cholesterol acceptor. Jurkat cells were labeled with [3H]cholesterol and resuspended in 0.08% MβCD. After a 3-min incubation at 37°C, cells were treated with 150 mU/ml B. cereus SMase (bSMase; closed squares) or left untreated (open squares). At different time intervals, the radioactivity that released into the extracellular medium was determined by liquid scintillation counting. Date shown are from one experiment with single point determinations, representative of at least five independent experiments. (B) Cells induced to undergo apoptosis (200 ng/ml; anti-CD95 mAb, closed symbols) or left untreated (open symbols) were pelleted after 1-, 2-, 3-, or 4-h incubation (indicated) and resuspended in medium containing 0.08% MβCD. After different incubation periods at 37°C, radioactivity which was released into the medium, was determined by liquid scintillation counting. Data are expressed as a percentage of radioactive cholesterol initially present in the cells and represent one out of three independent experiments with similar results.
Comment in
- Apoptosis and sphingomyelin hydrolysis. The flip side.
Green DR. Green DR. J Cell Biol. 2000 Jul 10;150(1):F5-7. doi: 10.1083/jcb.150.1.f5. J Cell Biol. 2000. PMID: 10893276 Free PMC article. No abstract available.
Similar articles
- Sorting of an internalized plasma membrane lipid between recycling and degradative pathways in normal and Niemann-Pick, type A fibroblasts.
Koval M, Pagano RE. Koval M, et al. J Cell Biol. 1990 Aug;111(2):429-42. doi: 10.1083/jcb.111.2.429. J Cell Biol. 1990. PMID: 2380243 Free PMC article. - Glucosylceramide synthase does not attenuate the ceramide pool accumulating during apoptosis induced by CD95 or anti-cancer regimens.
Tepper AD, Diks SH, van Blitterswijk WJ, Borst J. Tepper AD, et al. J Biol Chem. 2000 Nov 3;275(44):34810-7. doi: 10.1074/jbc.M005142200. J Biol Chem. 2000. PMID: 10945987 - Intracellular transport and metabolism of sphingomyelin.
Koval M, Pagano RE. Koval M, et al. Biochim Biophys Acta. 1991 Mar 12;1082(2):113-25. doi: 10.1016/0005-2760(91)90184-j. Biochim Biophys Acta. 1991. PMID: 2007175 Review. - Sphingomyelin hydrolysis during apoptosis.
Andrieu-Abadie N, Levade T. Andrieu-Abadie N, et al. Biochim Biophys Acta. 2002 Dec 30;1585(2-3):126-34. doi: 10.1016/s1388-1981(02)00332-3. Biochim Biophys Acta. 2002. PMID: 12531545 Review.
Cited by
- Microglial microvesicle secretion and intercellular signaling.
Turola E, Furlan R, Bianco F, Matteoli M, Verderio C. Turola E, et al. Front Physiol. 2012 May 22;3:149. doi: 10.3389/fphys.2012.00149. eCollection 2012. Front Physiol. 2012. PMID: 22661954 Free PMC article. - Apoptosis and sphingomyelin hydrolysis. The flip side.
Green DR. Green DR. J Cell Biol. 2000 Jul 10;150(1):F5-7. doi: 10.1083/jcb.150.1.f5. J Cell Biol. 2000. PMID: 10893276 Free PMC article. No abstract available. - Sphingomyelin Metabolism Is a Regulator of K-Ras Function.
van der Hoeven D, Cho KJ, Zhou Y, Ma X, Chen W, Naji A, Montufar-Solis D, Zuo Y, Kovar SE, Levental KR, Frost JA, van der Hoeven R, Hancock JF. van der Hoeven D, et al. Mol Cell Biol. 2018 Jan 16;38(3):e00373-17. doi: 10.1128/MCB.00373-17. Print 2018 Feb 1. Mol Cell Biol. 2018. PMID: 29158292 Free PMC article. - Sphingomyelin modulates the transbilayer distribution of galactosylceramide in phospholipid membranes.
Mattjus P, Malewicz B, Valiyaveettil JT, Baumann WJ, Bittman R, Brown RE. Mattjus P, et al. J Biol Chem. 2002 May 31;277(22):19476-81. doi: 10.1074/jbc.M201305200. Epub 2002 Mar 21. J Biol Chem. 2002. PMID: 11909867 Free PMC article.
References
- Anderson R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
- Andrieu N., Salvayre R., Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur. J. Biochem. 1996;236:738–745. - PubMed
- Anthony M.L., Zhao M., Brindle K.M. Inhibition of phosphatidylcholine biosynthesis following induction of apoptosis in HL-60 cells. J. Biol. Chem. 1999;274:19686–19692. - PubMed
- Boesen-de Cock J.G.R., Tepper A.D., de Vries E., van Blitterswijk W.J., Borst J. CD95 (Fas/APO-1) induces ceramide formation and apoptosis in the absence of a functional acid sphingomyelinase. J. Biol. Chem. 1998;273:7560–7565. - PubMed
- Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R.N. Ceramide synthase mediates daunorubicin-induced apoptosisan alternative mechanism for generating death signals. Cell. 1995;82:405–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous