Self-assembly of the Agrobacterium tumefaciens VirB11 traffic ATPase - PubMed (original) (raw)
Self-assembly of the Agrobacterium tumefaciens VirB11 traffic ATPase
S Rashkova et al. J Bacteriol. 2000 Aug.
Abstract
The Agrobacterium tumefaciens VirB11 ATPase is a component of a type IV transporter dedicated to T-DNA delivery to plant cells. In this study, we tested a prediction from genetic findings that VirB11 self-associates in vivo. A chimeric protein composed of VirB11 fused to the DNA binding domain of lambda cI repressor protein formed dimers, as shown by immunity of Escherichia coli to lambda superinfection. An allele encoding VirB11 fused at its C terminus to the green fluorescent protein (GFP) exerted strong negative dominance when synthesized in wild-type A. tumefaciens cells. Dominance was suppressed by overproduction of native VirB11, suggestive of titrating or competitive interactions between VirB11 and VirB11::GFP. In support of the titration model, a complex of native VirB11 and VirB11::GFP was recovered by precipitation with anti-GFP antibodies from detergent-solubilized A. tumefaciens cell extracts. VirB11 was shown by cI repressor fusion and immunoprecipitation assays to interact with VirB11 derivatives encoded by (i) 11 dominant negative alleles, (ii) recessive alleles bearing codon substitutions or deletions in the Walker A nucleotide binding motif, and (iii) alleles corresponding to the 5' and 3' halves of virB11. Further immunoprecipitation studies showed a hybrid protein composed of the N-terminal half of VirB11 fused to GFP interacted with mutant proteins exerting dominant effects and with a recessive Walker A deletion mutant (Delta GKT174-176). By contrast, a hybrid protein composed of the C-terminal half fused to GFP interacted with mutants exerting dominant effects but not the Walker A mutant protein. Together, these studies establish that VirB11 assembles as homomultimers in vivo via domains residing in each half of the protein. Furthermore, ATP binding appears to be critical for C-terminal interactions required for assembly of productive homomultimers.
Figures
FIG. 1
virB11.343::gfp dominance and suppression by VirB11 overproduction. A. tumefaciens strains expressing wild-type virB11, gfp, and/or virB11.343::gfp were assayed for virulence by inoculation onto wounded K. daigremontiana leaves. A348, wild type; PC1011, Δ_virB11_ mutant; PC2111, A348 merodiploid expressing virB11 and virB11.343::gfp from the Ti plasmid. Broad-host-range plasmids and relevant genes expressed: pXZB2 (P_lac_::virB11.343::gfp), pXZB63 (P_lac_::gfp), pED11 (P_virB_::virB11).
FIG. 2
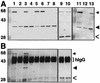
Coprecipitation of VirB11 and VirB11.343::GFP. (A) TX-100-solubilized proteins were subjected to SDS-PAGE and immunostaining with anti-VirB11 (lanes 1 to 8 and 11 to 13) or anti-GFP (lanes 9 and 10) antiserum. (B) Immunoprecipitates of protein extracts shown in panel A, immunoprecipitated with anti-GFP antiserum (lanes 1 to 4 and 7 to 10) or with preimmune serum (lanes 5 and 6). Lanes: 2, 4, and 12, wild-type A348 expressing virB11 (from pTi); 1, 5, and 11, A348(pXZB2) expressing virB11 (pTi) and virB11.343::gfp (IncP); 7 and 9, A348(pXZB63) expressing virB11 (pTi) and gfp (IncP); 3 and 6, the virB operon deletion strain, PC1000(pSR70), expressing virB11.343::gfp (IncP) and virB11 (IncP); 8 and 10, PC1000(pSR71) expressing virB11 (IncP) and gfp (IncP); 13, PC2111(pED11) expressing virB11 (pTi and IncP) and virB11.343::gfp (pTi). hIgG, immunoreactive heavy chain of IgG. Arrows indicate positions of VirB11::GFP, arrowheads denote VirB11, and open arrows denote GFP. Positions of molecular weight markers are shown at the left, with sizes in kilodaltons indicated.
FIG. 3
Cross-streak analysis of AG1688 expressing the chimera c_I::virB11. (A) Cross-streak analyses against λKH54. (B) Cross-streak analyses against λ_imm_21_c. Repressor constructs are indicated at the left, and immunity (imm) or sensitivity (sens) to phage is indicated at the right. Constructs and corresponding plasmids: cI (ind1), pJH157; cI′::virB11, pSR66; cI 1-102 (pKH101); vector, pZ150.
FIG. 4
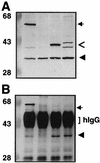
Coprecipitation of VirB11 and VirB11 truncation derivatives fused to GFP. (A) TX-100-solubilized proteins subjected to SDS-PAGE and immunoblotted with anti-VirB11 antiserum. (B) Immunoprecipitates of protein extracts shown in panel A after immunoprecipitation with anti-GFP antiserum. Lanes: 1, PC1000(pSR70) expressing virB11.343::gfp and virB11; 2, PC1000(pED11) expressing virB11; 3, PC1000(pSR81) expressing virB11[1-157]::gfp and virB11 (IncP plasmid); 4, PC1000(pSR82) expressing virB11[157-343]::gfp and virB11. hIgG, immunoreactive IgG heavy chain. Arrows indicate positions of VirB11.343::GFP, arrowheads identify VirB11, and open arrows identify VirB11 truncations fused to GFP. Positions of molecular weight markers are shown at the left, with sizes in kilodaltons indicated.
FIG. 5
Cross-streak analyses of AG1688 expressing _c_I repressor fusions to virB11 deletion derivatives. Repressor constructs are indicated at the left, and immunity (imm) or sensitivity (sens) to phage is indicated at the right. Constructs and corresponding plasmids from top to bottom: cI::VirB11, pSR66; cI′::VirB11[1-157], pSR61; VirB11[157-343], pSR62; cI′::VirB11[157-302], pSR63; cI′::VirB11[245-343], pSR64; cI::cIind1, pJH157; vector, pZ150.
Similar articles
- Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter.
Dang TA, Zhou XR, Graf B, Christie PJ. Dang TA, et al. Mol Microbiol. 1999 Jun;32(6):1239-53. doi: 10.1046/j.1365-2958.1999.01436.x. Mol Microbiol. 1999. PMID: 10383764 Free PMC article. - Role of Agrobacterium VirB11 ATPase in T-pilus assembly and substrate selection.
Sagulenko E, Sagulenko V, Chen J, Christie PJ. Sagulenko E, et al. J Bacteriol. 2001 Oct;183(20):5813-25. doi: 10.1128/JB.183.20.5813-5825.2001. J Bacteriol. 2001. PMID: 11566978 Free PMC article. - Suppression of mutant phenotypes of the Agrobacterium tumefaciens VirB11 ATPase by overproduction of VirB proteins.
Zhou XR, Christie PJ. Zhou XR, et al. J Bacteriol. 1997 Sep;179(18):5835-42. doi: 10.1128/jb.179.18.5835-5842.1997. J Bacteriol. 1997. PMID: 9294442 Free PMC article. - Characterization of membrane and protein interaction determinants of the Agrobacterium tumefaciens VirB11 ATPase.
Rashkova S, Spudich GM, Christie PJ. Rashkova S, et al. J Bacteriol. 1997 Feb;179(3):583-91. doi: 10.1128/jb.179.3.583-591.1997. J Bacteriol. 1997. PMID: 9006008 Free PMC article. - Agrobacterium tumefaciens VirB11 protein requires a consensus nucleotide-binding site for function in virulence.
Stephens KM, Roush C, Nester E. Stephens KM, et al. J Bacteriol. 1995 Jan;177(1):27-36. doi: 10.1128/jb.177.1.27-36.1995. J Bacteriol. 1995. PMID: 7798144 Free PMC article.
Cited by
- Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion.
Atmakuri K, Cascales E, Christie PJ. Atmakuri K, et al. Mol Microbiol. 2004 Dec;54(5):1199-211. doi: 10.1111/j.1365-2958.2004.04345.x. Mol Microbiol. 2004. PMID: 15554962 Free PMC article. - Agrobacterium tumefaciens oncogenic suppressors inhibit T-DNA and VirE2 protein substrate binding to the VirD4 coupling protein.
Cascales E, Atmakuri K, Liu Z, Binns AN, Christie PJ. Cascales E, et al. Mol Microbiol. 2005 Oct;58(2):565-79. doi: 10.1111/j.1365-2958.2005.04852.x. Mol Microbiol. 2005. PMID: 16194240 Free PMC article. - Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification.
Planet PJ, Kachlany SC, DeSalle R, Figurski DH. Planet PJ, et al. Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2503-8. doi: 10.1073/pnas.051436598. Proc Natl Acad Sci U S A. 2001. PMID: 11226268 Free PMC article. - Evidence for VirB4-mediated dislocation of membrane-integrated VirB2 pilin during biogenesis of the Agrobacterium VirB/VirD4 type IV secretion system.
Kerr JE, Christie PJ. Kerr JE, et al. J Bacteriol. 2010 Oct;192(19):4923-34. doi: 10.1128/JB.00557-10. Epub 2010 Jul 23. J Bacteriol. 2010. PMID: 20656905 Free PMC article. - Protein-protein interactions among Helicobacter pylori cag proteins.
Busler VJ, Torres VJ, McClain MS, Tirado O, Friedman DB, Cover TL. Busler VJ, et al. J Bacteriol. 2006 Jul;188(13):4787-800. doi: 10.1128/JB.00066-06. J Bacteriol. 2006. PMID: 16788188 Free PMC article.
References
- Burns D L. Biochemistry of type IV secretion. Curr Opin Microbiol. 1999;2:25–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources