Role of FliJ in flagellar protein export in Salmonella - PubMed (original) (raw)
Role of FliJ in flagellar protein export in Salmonella
T Minamino et al. J Bacteriol. 2000 Aug.
Abstract
We isolated and characterized spontaneous mutants with defects in the 147-amino-acid Salmonella protein FliJ, which is a cytoplasmic component of the type III flagellar export apparatus. These mutants, including ones with null mutations, have the ability to form swarms on motility agar plates after prolonged incubation at 30 degrees C; i.e., they display a leaky motile phenotype. One mutant, SJW277, which formed significantly bigger swarms than the others, encoded only the N-terminal 73 amino acids of FliJ, one-half of the protein. At 30 degrees C, overproduction of this mutant protein improved, to wild-type levels, both motility and the ability to export both rod/hook-type (FlgD; hook capping protein) and filament-type (FliC; flagellin) substrates. At 42 degrees C, however, export was inhibited, indicating that the mutant FliJ protein was temperature sensitive. Taking advantage of this, we performed temperature upshift experiments, which demonstrated that FliJ is directly required for the export of FliC. Co-overproduction of FliJ and either of two export substrates, FliE or FlgG, hindered their aggregation in the cytoplasm. We conclude that FliJ is a general component of the flagellar export apparatus and has a chaperone-like activity for both rod/hook-type and filament-type substrates.
Figures
FIG. 1
(A) Estimation of the total (dimer plus trimer) probability that a given residue of the 147-amino-acid wild-type sequence of FliJ will participate in an α-helical coiled-coil structure. The probability for residues 14 through 42 is ≥80%. Data were calculated by the MultiCoil program of Wolf et al. (24). In the schematic representation of the wild-type FliJ sequence (bottom), the region with a high probability of having coiled-coil structure is shaded. (B) Mutant FliJ proteins analyzed in this study. Sequence present is shown in solid bars, with the beginning and ending residues indicated. SJW104 has an in-frame deletion. SJW135 and SJW277 are nonsense mutants. The SJW104 and SJW277 FliJ products are referred to throughout this paper as FliJ(Δ13–24) and FliJ-N73, respectively.
FIG. 2
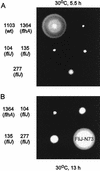
Swarming ability of fliJ mutants incubated at 30°C on semisolid tryptone agar plates for 5.5 h (A) or 13 h (B). All strain numbers carry the prefix SJW, which has been omitted for clarity. SJW1103 is wild type (wt) for motility and chemotaxis. SJW1364 is defective in FlhA, a component essential for export. The other strains are spontaneous fliJ mutants, all of which (most notably SJW277, encoding FliJ-N73) show some swarming ability by 13 h.
FIG. 3
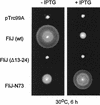
Swarming ability of the _fliJ_-null mutant SJW135 transformed with pTrc99A, pMM404 (wild-type [wt] FliJ), pRCJ104 [FliJ(Δ13–24)], or pRCJ277 (FliJ-N73). Transformants were incubated at 30°C for 6 h on semisolid tryptone agar plates with (+) or without (−) 0.1 mM IPTG.
FIG. 4
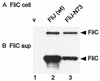
Effects of FliJ expression on intracellular levels of the rod/hook-type protein FlgD and the filament-type protein FliC (A, C, and E) and their export to the culture supernatant (sup) (B, D, and F). Strain SJW135gK (fliJ flgK::Tn_10_) was transformed with pTrc99A (vector [v]), pMM404 (wild-type [wt] FliJ), and either pRCJ277 (FliJ-N73) (A, B, E, and F) or pRCJ104 [FliJ(Δ13–24)] (C and D). Transformants were grown until late log phase in medium containing IPTG at the concentrations indicated. The samples were subjected to SDS-PAGE and immunoblotted with anti-FlgD (A to D) or anti-FliC (E and F). The positions of molecular mass markers (in kilodaltons) are shown on the left.
FIG. 5
Export properties of FliJ-N73 at 42°C. Strain SJW135gK (fliJ flgK::Tn_10_) was transformed with pTrc99A (vector [v]), pMM404 (wild-type [wt] FliJ), or pRCJ277 (FliJ-N73). Transformants were grown at 42°C to late log phase in medium containing IPTG at the concentrations indicated. The intracellular fractions (A) and culture supernatants (sup) (B) were subjected to SDS-PAGE and immunoblotted with anti-FlgD. The position of a molecular mass marker (in kilodaltons) is shown on the left.
FIG. 6
Temperature shift experiment to measure FliJ-mediated export of FliC at 42°C. SJW135gK (fliJ flgK::Tn_10_) cells carrying the pTrc99A vector (v), pMM404 (wild-type [wt] FliJ), or pRCJ277 (FliJ-N73) were grown at 30°C (in the presence of 1 mM IPTG for pRCJ277 only), washed, and grown at 42°C for 60 min in the absence of IPTG. The intracellular fractions (A) and culture supernatants (B) were subjected to SDS-PAGE and immunoblotted with anti-FliC antibody.
FIG. 7
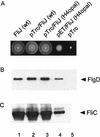
Steady-state intracellular levels of forms of FliJ in SJW135 (fliJ) cells carrying the plasmids pMM406 (N-His-tagged wild-type [wt] FliJ), pMM411 [N-His-tagged FliJ(Δ13–24)], or pMM410 (N-His-tagged FliJ-N73), induced at the IPTG concentrations shown and immunoblotted with anti-His antibody. The positions of molecular mass markers (in kilodaltons) are shown to the left.
FIG. 8
(A) Effect of FliJ expression on swarming of cells on semisolid tryptone agar at 30°C for 6 h. Colony 1, wild-type (wt) SJW1103/pTrc99A; colony 2, SJW1103/pMM404 (pTrc99A encoding wild-type FliJ); colony 3, SJW135 (fliJ)/pMM450 (pTrc99A encoding FliJ H4opal); colony 4, SJW135/pMM451 (pET22b encoding FliJ H4opal; colony 5, SJW135/pTrc99A. (B and C) Effect of FliJ expression on export of FlgD (hook capping protein) and FliC (flagellin) into the culture supernatant, detected in both cases with the appropriate antibody. Lanes 1, SJW1103gK (flgK)/pTrc99A; lanes 2, SJW1103gK/pMM404 (pTrc99A encoding wild-type FliJ); lanes 3, SJW135gK (fliJ flgK)/pMM450 (pTrc99A encoding FliJ H4opal); lanes 4, SJW135gK/pMM451 (pET22b encoding FliJ H4opal); lanes 5, SJW135gK/pTrc99A. No induction by IPTG was used.
FIG. 9
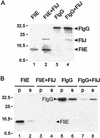
Inhibition by FliJ of aggregation of flagellar substrates FlgG (a rod protein) and FliE (a basal-body protein). SJW1368 [Δ(cheW-flhD)], a mutant defective in the master operon, was transformed with pTrc99A-based plasmid pMM1004 (encoding N-His-FLAG-FliE), pMM1004iJ (N-His-FLAG-FliE + N-His-FliJ), pMM203 (N-His-FLAG-FlgG), or pMM203iJ (N-His-FLAG-FlgG + N-His-FliJ). (A) Cells were grown in the absence of IPTG, washed, preincubated in fresh buffer, and radiolabeled with [35S]methionine for 5 min in the presence of IPTG. The sample was then boiled in SDS buffer, immunoprecipitated with anti-FLAG antibody, subjected to SDS-PAGE, and autoradiographed. (B) Cells were solubilized with B-PER reagent and fractionated by centrifugation at 20,800 × g for 5 min. The soluble (s) and pellet (p) fractions were then subjected to SDS-PAGE and immunoblotted with anti-FLAG antibody. The level of FliE is below the detection threshold in lanes 3 and 4. The positions of molecular mass markers (in kilodaltons) are shown on the left.
Similar articles
- Components of the Salmonella flagellar export apparatus and classification of export substrates.
Minamino T, Macnab RM. Minamino T, et al. J Bacteriol. 1999 Mar;181(5):1388-94. doi: 10.1128/JB.181.5.1388-1394.1999. J Bacteriol. 1999. PMID: 10049367 Free PMC article. - Interactions among components of the Salmonella flagellar export apparatus and its substrates.
Minamino T, MacNab RM. Minamino T, et al. Mol Microbiol. 2000 Mar;35(5):1052-64. doi: 10.1046/j.1365-2958.2000.01771.x. Mol Microbiol. 2000. PMID: 10712687 - Substrate specificity classes and the recognition signal for Salmonella type III flagellar export.
Hirano T, Minamino T, Namba K, Macnab RM. Hirano T, et al. J Bacteriol. 2003 Apr;185(8):2485-92. doi: 10.1128/JB.185.8.2485-2492.2003. J Bacteriol. 2003. PMID: 12670972 Free PMC article. - Protein export through the bacterial flagellar type III export pathway.
Minamino T. Minamino T. Biochim Biophys Acta. 2014 Aug;1843(8):1642-8. doi: 10.1016/j.bbamcr.2013.09.005. Epub 2013 Sep 21. Biochim Biophys Acta. 2014. PMID: 24064315 Review. - [Structure and function of the bacterial flagellar type III protein export system in Salmonella ].
Minamino T. Minamino T. Nihon Saikingaku Zasshi. 2015;70(3):351-64. doi: 10.3412/jsb.70.351. Nihon Saikingaku Zasshi. 2015. PMID: 26310179 Review. Japanese.
Cited by
- Interaction between FliJ and FlhA, components of the bacterial flagellar type III export apparatus.
Ibuki T, Uchida Y, Hironaka Y, Namba K, Imada K, Minamino T. Ibuki T, et al. J Bacteriol. 2013 Feb;195(3):466-73. doi: 10.1128/JB.01711-12. Epub 2012 Nov 16. J Bacteriol. 2013. PMID: 23161028 Free PMC article. - The HP0256 gene product is involved in motility and cell envelope architecture of Helicobacter pylori.
Douillard FP, Ryan KA, Lane MC, Caly DL, Moore SA, Penn CW, Hinds J, O'Toole PW. Douillard FP, et al. BMC Microbiol. 2010 Apr 8;10:106. doi: 10.1186/1471-2180-10-106. BMC Microbiol. 2010. PMID: 20377912 Free PMC article. - Flagellar Basal Body Structural Proteins FlhB, FliM, and FliY Are Required for Flagellar-Associated Protein Expression in Listeria monocytogenes.
Cheng C, Wang H, Ma T, Han X, Yang Y, Sun J, Chen Z, Yu H, Hang Y, Liu F, Fang W, Jiang L, Cai C, Song H. Cheng C, et al. Front Microbiol. 2018 Feb 13;9:208. doi: 10.3389/fmicb.2018.00208. eCollection 2018. Front Microbiol. 2018. PMID: 29487588 Free PMC article. - Mechanism of type-III protein secretion: Regulation of FlhA conformation by a functionally critical charged-residue cluster.
Erhardt M, Wheatley P, Kim EA, Hirano T, Zhang Y, Sarkar MK, Hughes KT, Blair DF. Erhardt M, et al. Mol Microbiol. 2017 Apr;104(2):234-249. doi: 10.1111/mmi.13623. Epub 2017 Feb 28. Mol Microbiol. 2017. PMID: 28106310 Free PMC article. - Analysis of the cytoplasmic domains of Salmonella FlhA and interactions with components of the flagellar export machinery.
McMurry JL, Van Arnam JS, Kihara M, Macnab RM. McMurry JL, et al. J Bacteriol. 2004 Nov;186(22):7586-92. doi: 10.1128/JB.186.22.7586-7592.2004. J Bacteriol. 2004. PMID: 15516571 Free PMC article.
References
- Fan F, Ohnishi K, Francis N R, Macnab R M. The FliP and FliR proteins of Salmonella typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol. 1997;26:1035–1046. - PubMed
- Fraser G M, Bennett J C Q, Hughes C. Substrate-specific binding of hook-associated proteins by FlgN and FliT, putative chaperones for flagellum assembly. Mol Microbiol. 1999;32:569–580. - PubMed
- Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases