Monoclonal antibodies - PubMed (original) (raw)
Review
Monoclonal antibodies
P N Nelson et al. Mol Pathol. 2000 Jun.
Abstract
Monoclonal antibodies are essential tools for many molecular immunology investigations. In particular, when used in combination with techniques such as epitope mapping and molecular modelling, monoclonal antibodies enable the antigenic profiling and visualisation of macromolecular surfaces. In addition, monoclonal antibodies have become key components in a vast array of clinical laboratory diagnostic tests. Their wide application in detecting and identifying serum analytes, cell markers, and pathogenic agents has largely arisen through the exquisite specificity of these unique reagents. Furthermore, the continuous culture of hybridoma cells that produce these antibodies offers the potential of an unlimited supply of reagent. In essence, when compared with the rather limited supply of polyclonal antibody reagents, the feature of a continuous supply enables the standardisation of both the reagent and the assay technique. Clearly, polyclonal and monoclonal antibodies have their advantages and disadvantages in terms of generation, cost, and overall applications. Ultimately, monoclonal antibodies are only produced when necessary because their production is time consuming and frustrating, although greatly rewarding (at least most of the time!). This is especially apparent when a monoclonal antibody can be applied successfully in a routine pathology laboratory or can aid in the clinical diagnosis and treatment of patients. In this article, the generation and application of monoclonal antibodies are demystified to enable greater understanding and hopefully formulate novel ideas for clinicians and scientists alike.
Figures
Figure 1
Schematic representation of an antibody molecule highlighting the “Y” shaped structure.
Figure 2
Five stages of generating a murine monoclonal antibody (MAb). (A) Immunisation, illustrating tail bleeds from mice immunised with Epstein-Barr virus (EBV) latent membrane protein 1 multiple antigenic synthetic peptide. (B) Fusion and selection, showing hybridoma PNG312G. (C) Screening, highlighting reactivity of MAb PNG211D against human osteoclastoma. (D) Characterisation, epitope mapping of MAb A57H against human IgG Fc. (E) Further developments, molecular modelling of monoclonal antibody A57H revealing an epitope (red) in the CH3 domain of IgG. CFA, complete Freund's adjuvant; ELISA, enzyme linked immunosorbent assay; HA, haemagglutination; HAT, hypoxanthine, aminopterin, and thymidine; HT, hypoxanthine and thymidine; IC, immunochemistry; IFA, incomplete Freund's adjuvant; PEG, polyethylene glycol; WBLOT, western blotting.
Figure 3
A monoclonal antibody directed against cytokeratin demonstrates micrometastases in smooth muscle of large bowel.
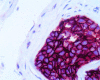
Figure 4
The use of a monoclonal antibody in the immunocytochemical detection of high HER-2/neu expression in a metastatic deposit of breast cancer.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.
Shabaninejad H, Kenny RP, Robinson T, Stoniute A, O'Keefe H, Still M, Thornton C, Pearson F, Beyer F, Meader N. Shabaninejad H, et al. Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- A placebo-controlled, double-blind, dose-escalation study to assess the safety, tolerability and pharmacokinetics/pharmacodynamics of single and multiple intravenous infusions of AZD9773 in patients with severe sepsis and septic shock.
Morris PE, Zeno B, Bernard AC, Huang X, Das S, Edeki T, Simonson SG, Bernard GR. Morris PE, et al. Crit Care. 2012 Feb 17;16(1):R31. doi: 10.1186/cc11203. Crit Care. 2012. PMID: 22340283 Free PMC article. Clinical Trial. - Epitope mapping of a monoclonal antibody against human thrombin by H/D-exchange mass spectrometry reveals selection of a diverse sequence in a highly conserved protein.
Baerga-Ortiz A, Hughes CA, Mandell JG, Komives EA. Baerga-Ortiz A, et al. Protein Sci. 2002 Jun;11(6):1300-8. doi: 10.1110/ps.4670102. Protein Sci. 2002. PMID: 12021429 Free PMC article. - Generation and Characterization of an scFv Directed against Site II of Rabies Glycoprotein.
Aavula SM, Nimmagadda SV, Biradhar N, Sula S, Chandran D, Lingala R, Villuppanoor SA. Aavula SM, et al. Biotechnol Res Int. 2011;2011:652147. doi: 10.4061/2011/652147. Epub 2011 Oct 5. Biotechnol Res Int. 2011. PMID: 22007309 Free PMC article. - Epitope mapping of human VWF A3 recognized by monoclonal antibody SZ-123 and SZ-125 using MALDI mass spectrometry.
Jiang M, Zhao Y, Shen F, Wang F, He Y, Ruan C. Jiang M, et al. Int J Hematol. 2011 Sep;94(3):241-247. doi: 10.1007/s12185-011-0904-x. Epub 2011 Aug 6. Int J Hematol. 2011. PMID: 21822587 - Development of Monoclonal Antibody against PirB and Establishment of a Colloidal Gold Immunochromatographic Assay for the Rapid Detection of AHPND-Causing Vibrio.
Dong X, Xie J, Wang L, Li X, Lou H, Wang G, Huang J. Dong X, et al. Animals (Basel). 2024 May 29;14(11):1600. doi: 10.3390/ani14111600. Animals (Basel). 2024. PMID: 38891648 Free PMC article.
References
- Nelson PN, Westwood OM, Jefferis R, et al. Characterisation of anti-IgG monoclonal antibody A57H by epitope mapping. Biochem Soc Trans 1997;25:373. - PubMed
- Nelson PN, Fletcher SM, De Lange GG, et al. Evaluation of monoclonal antibodies with putative specificity for human IgG allotypes. Vox Sang 1990;59:190–7. - PubMed
- Nelson PN, Fletcher SM, MacDonald D, et al. Assay restriction profiles of three monoclonal antibodies recognizing the G3m(u) allotype: development of an allotype specific assay. J Immunol Methods 1991;138:57–64. - PubMed
- Blottiere HM, Daculsi G, Anegon I, et al. Utilization of activated U937 monocytic cells as a model to evaluate biocompatibility and biodegradation of synthetic calcium phosphate. Biomaterials 1995;16:497–503. - PubMed
- Hudson PJ. Recombinant antibody constructs in cancer therapy. Curr Opin Immunol 1999;11:548–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources