Distinct roles of two Yth1p domains in 3'-end cleavage and polyadenylation of yeast pre-mRNAs - PubMed (original) (raw)
Distinct roles of two Yth1p domains in 3'-end cleavage and polyadenylation of yeast pre-mRNAs
S M Barabino et al. EMBO J. 2000.
Abstract
Yth1p is the yeast homologue of the 30 kDa subunit of mammalian cleavage and polyadenylation specificity factor (CPSF). The protein is part of the cleavage and polyadenylation factor CPF, which includes cleavage factor II (CF II) and polyadenylation factor I (PF I), and is required for both steps in pre-mRNA 3'-end processing. Yth1p is an RNA-binding protein that was previously shown to be essential for polyadenylation. Here, we demonstrate that Yth1p is also required for the cleavage reaction and that two protein domains have distinct roles in 3'-end processing. The C-terminal part is required in polyadenylation to tether Fip1p and poly(A) polymerase to the rest of CPF. A single point mutation in the highly conserved second zinc finger impairs both cleavage and polyadenylation, and affects the ability of Yth1p to interact with the pre-mRNA and other CPF subunits. Finally, we find that Yth1p binds to CYC1 pre-mRNA in the vicinity of the cleavage site. Our results indicate that Yth1p is important for the integrity of CPF and participates in the recognition of the cleavage site.
Figures
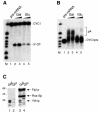
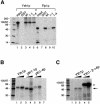
Fig. 1. In vivo depletion of Yth1 protein affects both pre-mRNA 3′-end processing steps. (A) Cleavage assay with CYC1 pre-mRNA. (B) Polyadenylation assay with pre-cleaved CYC1 RNA, which ends at the natural cleavage site. Cells expressing a ubiquitin–R-Yth1 fusion protein under the control of a UAS-GAL-CYC1 hybrid promoter (pGUR-YTH1) were grown in either galactose- or glucose–containing medium. Increasing amounts of extract (10 and 20 µg, respectively) prepared from these cells (galactose, lanes 2 and 3; glucose, lanes 4 and 5) were incubated with the RNA under standard assay conditions for 1 h. The position of the precursors (lane 1), the 5′ cleavage product and the polyadenylated RNA are indicated. _Hpa_II-digested pBR322 fragments served as marker (M). They range from 309 to160 nucleotides in (A) and from 309 to 180 nucleotides in (B). (C) Western blot of extracts prepared from cells expressing a ubiquitin–R-Yth1 fusion protein under the control of a UAS-GAL-CYC1 hybrid promoter (pGUR-YTH1), which were grown in either galactose- or glucose-containing medium (lanes 1 and 3, and 2 and 4, respectively). Ten micrograms of extract were fractionated on a 12% SDS–polyacrylamide gel, blotted onto a nitrocellulose membrane and probed first with α-Yth1p, and subsequently with α-Fip1p and α-Rna15p antibodies.
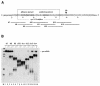
Fig. 2. Mutagenesis in the highly conserved second zinc finger of Yth1p. In the schematic alignment of Yth1p and CPSF 30K, zinc fingers 1, 3 and 5 are represented by black boxes and zinc fingers 2 and 4 by striped boxes. The C-terminal zinc knuckle motif of CPSF 30K is represented by a stippled box. In the amino acid sequence alignment of the second and fourth zinc fingers of CPSF 30K and Yth1p, the cysteine and histidine residues are indicated white on black; all other identical residues are boxed. Bos taurus CPSF 30K (DDBJ/EMBL/GenBank accession No. U96448; Barabino et al., 1997), S.cerevisiae YTH1 (systematic name: YPR107c; Barabino et al., 1997). The results from the site-directed mutagenesis of zinc finger 2 are summarized in the table below the alignments.
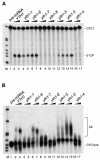
Fig. 3. YTH1 mutant extracts are impaired in cleavage and polyadenylation. (A) Cleavage assay. (B) Polyadenylation assay. Extracts prepared from wild-type cells (lanes 2 and 3) and from yth1 mutant strains were added to the reactions as indicated on top of each lane. The position of the precursors (lane 1) and either the 5′ cleavage product or of the polyadenylated RNA are indicated. End-labelled _Hpa_II-digested pBR322 fragments served as marker (M).
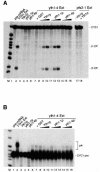
Fig. 4. Reconstitution of cleavage activity in yth1-4 extract by complementation with recombinant Yth1 protein. (A) Cleavage assay. (B) Polyadenylation assay. Wild-type YTH1 yeast extract (lane 2) served as positive control. Two hundred nanograms of recombinant GST–Yth1p, GST–Yth1-1p or GST–Yth1-4p were incubated with the pre-mRNA (lanes 3–5). yth1-4 mutant extract was incubated with CYC1 RNA either alone (lane 6) or in combination with GST (lane 7), 5–200 ng of GST–Yth1p (lanes 8–10), GST–Yth1-1p (lanes 11–13) or GST–Yth1-4p (lanes 14–16). In (A), an aliquot of a pfs2-1 mutant extract was tested either alone or in combination with 200 ng of Yth1p (lanes 17 and 18, respectively). The position of the precursors, the 5′- and the 3′-cleavage products and the polyadenylated reaction products is indicated on the right. End-labelled _Hpa_II-digested pBR322 fragments served as marker (M).
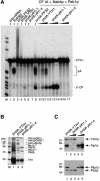
Fig. 5. Mutations in Yth1p affect the integrity of the CPF complex. (A) Reconstitution of in vitro pre-mRNA 3′-end processing with the protein complexes associated with wild-type and mutant Yth1p. The multiprotein complexes associated with either protein A–TEV-tagged Pfs2p, Yth1p, Yth1-1p or Yth1-4p were isolated by batch adsorption to IgG–agarose and cleavage with TEV protease as described in Materials and methods. Comparable amounts of the different IgG eluates were added to the coupled cleavage and polyadenylation assay as indicated on top of each lane. In lanes 8–17, the eluates were combined with a mixture of purified CF IA, 10 ng of Nab4p/Hrp1p and 100 ng of Pab1p in order to reconstitute 3′-end processing in vitro. A wild-type yeast extract served as positive control (lane 2). The position of the precursor (lane 1), the 5′ cleavage product and the polyadenylated RNA is indicated. End-labelled _Hpa_II-digested pBR322 fragments served as marker (M). (B) The CF II–PF I complex co-purifies with protein A-tagged Yth1 protein. The multiprotein complexes associated with protein A–TEV-tagged wild-type or mutant Yth1p were isolated from extracts prepared from strains SB16 (wild-type, lane1), SB17 (yth1-1, lane 2) and SB18 (yth1-4, lane 3). The isolated material was analysed on an SDS–polyacrylamide gel, which was subsequently stained with silver. About six times more of the wild-type Yth1 eluate was fractionated on the gel than of the mutant eluates. (C) Immunoblot analysis of the IgG eluates prepared from SB16 (lane 1), SB17 (yth1-1, lanes 2 and 3) and SB18 (lanes 4 and 5) extracts. The eluates were fractionated on a 10% SDS–polyacrylamide gel, blotted on a nitrocellulose membrane and probed with α-Ysh1p and α-Fip1p antibodies (top), or α-Pap1p and α-Pfs2p antibodies. The sizes of protein standards (in kilodaltons) are indicated on the left.
Fig. 6. Mutations in Yth1p impair protein–protein and RNA–protein interactions. (A) Pull-down experiment with glutathione–Sepharose. GST–tagged Yth1, Yth1-1 and Yth1-4 proteins were tested for interaction with either in vitro translated, 35S-labelled Ysh1p (lanes 3–5) or Fip1p (lanes 8–10). Lanes 1 and 6, 5% of the inputs; lanes 2 and 7, GST bound to glutathione–Sepharose. (B) UV cross-linking to CYC1 RNA. Increasing amounts of recombinant Yth1, Yth1-1 and Yth1-4 proteins were incubated with 100 fmol of pre-mRNA as described. To verify that comparable amounts of each protein had been loaded, the gel was first stained with Coomassie Blue. The amount of cross-linked protein was quantified as described in Materials and methods. Lanes 1, 3 and 5, 100 ng; lanes 2, 4 and 6, 200 ng; lane 7, pre-mRNA incubated without protein. (C) UV cross-linking with Yth1-2→4 protein to CYC1 RNA. Increasing amounts of recombinant Yth1 and Yth1-2→4 proteins were incubated with 150 fmol of pre-mRNA as described. An asterisk indicates a band that does not correspond to Yth1-2→4p as assessed by western blot analysis. The band corresponding to Yth1p is indicated by an arrowhead. Lanes 2 and 4, 50 ng; lanes 3 and 5, 100 ng; lane 1, pre-mRNA incubated without protein.
Fig. 7. Yth1 protein binds in the vicinity of the cleavage site. (A) Sequence of the ‘short’ CYC1 substrate (see Materials and methods) and position of the antisense DNA oligonucleotides #7–14. The nucleotides that define the efficiency and positioning elements (Russo et al., 1991) are indicated in bold. The region encompassing the deletion in the cyc1-512 substrate is boxed. Vector sequences are indicated in lower case. (B) RNase H protection assay with recombinant Yth1p and ‘short’ CYC1 pre-mRNA. The pre-mRNA substrate was pre-incubated either in the absence (–) or in the presence (+) of ∼200 ng of recombinant Yth1 protein before addition of the corresponding DNA oligonucleotide (indicated at the top) and RNase H. End-labelled _Hpa_II-digested pBR322 fragments served as marker (M).
Similar articles
- A multisubunit 3' end processing factor from yeast containing poly(A) polymerase and homologues of the subunits of mammalian cleavage and polyadenylation specificity factor.
Preker PJ, Ohnacker M, Minvielle-Sebastia L, Keller W. Preker PJ, et al. EMBO J. 1997 Aug 1;16(15):4727-37. doi: 10.1093/emboj/16.15.4727. EMBO J. 1997. PMID: 9303317 Free PMC article. - Human pre-mRNA cleavage factor II(m) contains homologs of yeast proteins and bridges two other cleavage factors.
de Vries H, Rüegsegger U, Hübner W, Friedlein A, Langen H, Keller W. de Vries H, et al. EMBO J. 2000 Nov 1;19(21):5895-904. doi: 10.1093/emboj/19.21.5895. EMBO J. 2000. PMID: 11060040 Free PMC article. - The WD-repeat protein pfs2p bridges two essential factors within the yeast pre-mRNA 3'-end-processing complex.
Ohnacker M, Barabino SM, Preker PJ, Keller W. Ohnacker M, et al. EMBO J. 2000 Jan 4;19(1):37-47. doi: 10.1093/emboj/19.1.37. EMBO J. 2000. PMID: 10619842 Free PMC article. - The end of the message: 3'-end processing leading to polyadenylated messenger RNA.
Wahle E. Wahle E. Bioessays. 1992 Feb;14(2):113-8. doi: 10.1002/bies.950140208. Bioessays. 1992. PMID: 1575710 Review. - A comparison of mammalian and yeast pre-mRNA 3'-end processing.
Keller W, Minvielle-Sebastia L. Keller W, et al. Curr Opin Cell Biol. 1997 Jun;9(3):329-36. doi: 10.1016/s0955-0674(97)80004-x. Curr Opin Cell Biol. 1997. PMID: 9159082 Review.
Cited by
- Structure of a nucleotide-bound Clp1-Pcf11 polyadenylation factor.
Noble CG, Beuth B, Taylor IA. Noble CG, et al. Nucleic Acids Res. 2007;35(1):87-99. doi: 10.1093/nar/gkl1010. Epub 2006 Dec 6. Nucleic Acids Res. 2007. PMID: 17151076 Free PMC article. - Functional analysis of yeast snoRNA and snRNA 3'-end formation mediated by uncoupling of cleavage and polyadenylation.
Morlando M, Greco P, Dichtl B, Fatica A, Keller W, Bozzoni I. Morlando M, et al. Mol Cell Biol. 2002 Mar;22(5):1379-89. doi: 10.1128/MCB.22.5.1379-1389.2002. Mol Cell Biol. 2002. PMID: 11839805 Free PMC article. - Dynamics in Fip1 regulate eukaryotic mRNA 3' end processing.
Kumar A, Yu CWH, Rodríguez-Molina JB, Li XH, Freund SMV, Passmore LA. Kumar A, et al. Genes Dev. 2021 Nov 1;35(21-22):1510-1526. doi: 10.1101/gad.348671.121. Epub 2021 Sep 30. Genes Dev. 2021. PMID: 34593603 Free PMC article. - Efficient mRNA polyadenylation requires a ubiquitin-like domain, a zinc knuckle, and a RING finger domain, all contained in the Mpe1 protein.
Lee SD, Moore CL. Lee SD, et al. Mol Cell Biol. 2014 Nov;34(21):3955-67. doi: 10.1128/MCB.00077-14. Epub 2014 Aug 18. Mol Cell Biol. 2014. PMID: 25135474 Free PMC article.
References
- Bachmair A., Finley,D. and Varshavsky,A. (1986) In vivo half-life of a protein is a function of its amino-terminal residue. Science, 234, 179–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous