Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins - PubMed (original) (raw)
Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins
L S Van Kirk et al. Infect Immun. 2000 Aug.
Abstract
Actin-based motility (ABM) is a mechanism for intercellular spread that is utilized by vaccinia virus and the invasive bacteria within the genera Rickettsia, Listeria, and Shigella. Within the Rickettsia, ABM is confined to members of the spotted fever group (SFG), such as Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Infection by each agent induces the polymerization of host cell actin to form the typical F (filamentous)-actin comet tail. Assembly of the actin tail propels the pathogen through the host cytosol and into cell membrane protrusions that can be engulfed by neighboring cells, initiating a new infectious cycle. Little is known about the structure and morphogenesis of the Rickettsia rickettsii actin tail relative to Shigella and Listeria actin tails. In this study we examined the ultrastructure of the rickettsial actin tail by confocal, scanning electron, and transmission electron microscopy. Confocal microscopy of rhodamine phalloidin-stained infected Vero cells revealed the typhus group rickettsiae, Rickettsia prowazekii and Rickettsia typhi, to have no actin tails and short (approximately 1- to 3-micrometer) straight or hooked actin tails, respectively. The SFG rickettsia, R. rickettsii, displayed long actin tails (>10 micrometer) that were frequently comprised of multiple, distinct actin bundles, wrapping around each other in a helical fashion. Transmission electron microscopy, in conjunction with myosin S1 subfragment decoration, revealed that the individual actin filaments of R. rickettsii tails are >1 micrometer long, arranged roughly parallel to one another, and oriented with the fast-growing barbed end towards the rickettsial pole. Scanning electron microscopy of intracellular rickettsiae demonstrated R. rickettsii to have polar associations of cytoskeletal material and R. prowazekii to be devoid of cytoskeletal interactions. By indirect immunofluorescence, both R. rickettsii and Listeria monocytogenes actin tails were shown to contain the cytoskeletal proteins vasodilator-stimulated phosphoprotein profilin, vinculin, and filamin. However, rickettsial tails lacked ezrin, paxillin, and tropomyosin, proteins that were associated with actin tails of cytosolic or protrusion-bound Listeria. The unique ultrastructural and compositional characteristics of the R. rickettsii actin tail suggest that rickettsial ABM is mechanistically different from previously described microbial ABM systems.
Figures
FIG. 1
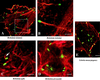
Actin tail phenotypes of rickettsiae and comparison to tails of L. monocytogenes. Dual fluorescent staining of intracellular bacteria and F-actin was conducted on Vero cells. F-actin was stained with rhodamine phalloidin (red), and intracellular bacteria were stained by indirect immunofluorescence (green). Cells were visualized by laser scanning confocal microscopy. (A) R. rickettsii showing long actin tails that are frequently comprised of multiple, twisting, distinct F-actin bundles. (B) High magnification of an R. rickettsii actin tail in panel A comprised of two F-actin bundles. (C) Truncated hook-shaped tail of R. typhi (arrow). (D) R. prowazekii with no actin tails. (E) Actin comet tails of L. monocytogenes. Bars, 5 μm.
FIG. 2
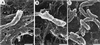
SEM of Vero cells infected with R. rickettsii or R. prowazekii. Cells were fixed and dry cleaved to expose the cell interior. (A and B) R. rickettsii with a polar stalk of cytoskeletal material. Note the organism undergoing binary fission with only one daughter cell associated with cytoskeletal material (B). (C) R. prowazekii devoid of polar cytoskeletal stalks. Bars, 0.5 μm.
FIG. 3
Ultrastructure of the R. rickettsii actin tail as viewed by TEM. (A) Sections of Vero cells infected with R. rickettsii showing a bilateral association of bundles of long actin filaments that appear to be minimally cross-linked. (B) Myosin S1 subfragment decoration of the rickettsial actin tail depicting long, parallel actin filaments. (C) High magnification of myosin S1 subfragment decorated tail filaments showing the fast-growing barbed ends of filaments oriented towards the rickettsial surface. A decorated actin filament, designated with an asterisk at the barbed end, is shown in the inset. Individual S1 subunits are demarcated with white lines to highlight the directional binding of this protein. Bars, 0.5 μm.
FIG. 4
Protrusion formation and cell-to-cell spread by R. rickettsii in Vero cells. (A) Thin section of rickettsia-containing protrusion. The plasma membrane of the infected cell and the adjacent uninfected cell are clearly visible. The actin tail has been grazed in this thin section. (B) Protrusion containing two rickettsiae that extends a few micrometers from the cell surface. The cup-shaped beginning of the accompanying actin tail is designated with an arrow. (C) SEM of R. rickettsii in a short protrusion that has collapsed to the cell surface. Bars, 0.5 μm.
FIG. 5
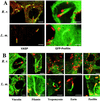
Fluorescence localization of the cytoskeletal proteins VASP, profilin, vinculin, filamin, tropomyosin, ezrin, and paxillin in fixed Vero cells infected with R. rickettsii (R. r.) or L. monocytogenes (L. m.). Images were collected using a confocal laser scanning microscope. Cytoskeletal proteins, with the exception of profilin, were labeled by indirect immunofluorescence by using specific monoclonal antibodies. Profilin was localized by transiently expressing GFP-profilin in infected cells as described in Materials and Methods. Intracellular bacteria were counterstained by indirect immunofluorescence. (A) VASP labeling (red) is diffusely dispersed throughout the actin tail of R. rickettsii (green), whereas labeling is concentrated to one pole of Listeria (green). (Note the rickettsiae apparently in the process of penetrating the nuclear membrane.) GFP-profilin (green) is similarly dispersed throughout the actin tail of R. rickettsii (red), in this case intranuclear rickettsiae, whereas GFP-profilin is primarily localized to one pole of Listeria (red) and the beginning of the actin tails. (B) Vinculin and filamin (green) were detected throughout the length of tails of R. rickettsii (red) and Listeria (red). Tropomyosin, ezrin, and paxillin (green) were detected in tails of cytoplasmic or protrusion-bound Listeria (green) but not tails of R. rickettsii (red). Bars, 5 μm.
Similar articles
- Rickettsial actin-based motility: behavior and involvement of cytoskeletal regulators.
Heinzen RA. Heinzen RA. Ann N Y Acad Sci. 2003 Jun;990:535-47. doi: 10.1111/j.1749-6632.2003.tb07424.x. Ann N Y Acad Sci. 2003. PMID: 12860687 - Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells.
Heinzen RA, Hayes SF, Peacock MG, Hackstadt T. Heinzen RA, et al. Infect Immun. 1993 May;61(5):1926-35. doi: 10.1128/iai.61.5.1926-1935.1993. Infect Immun. 1993. PMID: 8478082 Free PMC article. - Dynamics of actin-based movement by Rickettsia rickettsii in vero cells.
Heinzen RA, Grieshaber SS, Van Kirk LS, Devin CJ. Heinzen RA, et al. Infect Immun. 1999 Aug;67(8):4201-7. doi: 10.1128/IAI.67.8.4201-4207.1999. Infect Immun. 1999. PMID: 10417192 Free PMC article. - Molecular mechanisms of cell-cell spread of intracellular bacterial pathogens.
Ireton K. Ireton K. Open Biol. 2013 Jul 17;3(7):130079. doi: 10.1098/rsob.130079. Open Biol. 2013. PMID: 23864553 Free PMC article. Review. - Some contributions of electron microscopy to the study of the rickettsiae.
Silverman DJ. Silverman DJ. Eur J Epidemiol. 1991 May;7(3):200-6. doi: 10.1007/BF00145667. Eur J Epidemiol. 1991. PMID: 1909242 Review.
Cited by
- Hijacking Host Cell Highways: Manipulation of the Host Actin Cytoskeleton by Obligate Intracellular Bacterial Pathogens.
Colonne PM, Winchell CG, Voth DE. Colonne PM, et al. Front Cell Infect Microbiol. 2016 Sep 22;6:107. doi: 10.3389/fcimb.2016.00107. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27713866 Free PMC article. Review. - Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park.
Simser JA, Palmer AT, Fingerle V, Wilske B, Kurtti TJ, Munderloh UG. Simser JA, et al. Appl Environ Microbiol. 2002 Sep;68(9):4559-66. doi: 10.1128/AEM.68.9.4559-4566.2002. Appl Environ Microbiol. 2002. PMID: 12200314 Free PMC article. - Rickettsia-macrophage interactions: host cell responses to Rickettsia akari and Rickettsia typhi.
Radulovic S, Price PW, Beier MS, Gaywee J, Macaluso JA, Azad A. Radulovic S, et al. Infect Immun. 2002 May;70(5):2576-82. doi: 10.1128/IAI.70.5.2576-2582.2002. Infect Immun. 2002. PMID: 11953398 Free PMC article. - Expression analysis of the T-cell-targeting chemokines CXCL9 and CXCL10 in mice and humans with endothelial infections caused by rickettsiae of the spotted fever group.
Valbuena G, Bradford W, Walker DH. Valbuena G, et al. Am J Pathol. 2003 Oct;163(4):1357-69. doi: 10.1016/S0002-9440(10)63494-3. Am J Pathol. 2003. PMID: 14507644 Free PMC article. - Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility.
Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, Takeshita S, Heuser J, Welch MD, Brown EJ. Stamm LM, et al. J Exp Med. 2003 Nov 3;198(9):1361-8. doi: 10.1084/jem.20031072. J Exp Med. 2003. PMID: 14597736 Free PMC article.
References
- Bershadsky A D, Vasiliev J M, editors. Cytoskeleton. New York, N.Y: Plenum Publishing Corp.; 1988.
- Chakraborty T, Ebel F, Domann E, Niebuhr K, Gerstel B, Pistor S, Temm-Grove C J, Jockusch B M, Reinhard M, Walter U, Wehland J. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995;14:1314–1321. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources