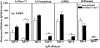B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection - PubMed (original) (raw)
B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection
N Baumgarth et al. J Exp Med. 2000.
Abstract
We have studied the role of secreted immunoglobulin (Ig)M in protection from infection with influenza virus and delineated the relative contributions of B-1 versus B-2 cell-derived IgM in this process. Mice deficient in secreted IgM but capable of expressing surface IgM and secreting other Ig classes show significantly reduced virus clearance and survival rates compared with wild-type controls. Irradiation chimeras in which only either B-1 or B-2 cells lack the ability to secrete IgM show mortality rates similar to those of mice in which neither B-1 nor B-2 cells secrete IgM. Dependence on both sources of IgM for survival is partially explained by findings in allotype chimeras that broadly cross-reactive B-1 cell-derived natural IgM is present before infection, whereas virus strain-specific, B-2 cell-derived IgM appears only after infection. Furthermore, lack of IgM secreted from one or both sources significantly impairs the antiviral IgG response. Reconstitution of chimeras lacking B-1 cell-derived IgM only with IgM-containing serum from noninfected mice improved both survival rates and serum levels of virus-specific IgG. Thus, virus-induced IgM must be secreted in the presence of natural IgM for efficient induction of specific IgG and for immune protection, identifying B-1 and B-2 cell-derived IgM antibodies as nonredundant components of the antiviral response.
Figures
Figure 1
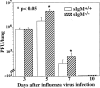
Increased influenza virus–induced mortality in mice lacking sIgM. Groups of 2- (n = 14; left panel) and 3-mo-old (n = 10; right panel) sIgM−/− and sIgM+/+ mice were infected with influenza virus Mem71, and survival was monitored daily. Percentages of survival are shown as a function of time.
Figure 3
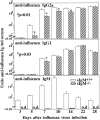
Impaired virus-specific IgG response in the absence of sIgM. Groups of 3-mo-old sIgM−/− and sIgM+/+ mice (n = 10) were infected with influenza virus Mem71. Mice were bled at indicated times, and the titers of influenza virus–specific IgM, IgG1, and IgG2a were measured by ELISA. Arbitrary units of antivirus Ig per milliliter were determined by comparison to a standard hyperimmune serum.
Figure 2
Reduced virus clearance in the lungs of sIgM−/− mice. Groups of 3-mo-old sIgM−/− and sIgM+/+ mice (10–12 per time point) were infected with influenza virus Mem71, and lung homogenates were prepared at indicated times after infection. Lung virus titers were determined by MDCK cell plaque assay. Statistical analysis was determined using the Wilcoxon Rank Test.
Figure 5
Adoptive cell transfer of bone marrow and PerC cells results in B-1/B-2 chimerism. Lethally irradiated C57BL/6 mice were reconstituted with bone marrow cells from C57BL/6 (Ighb) and PerC cells from congenic B6.C20 (Igha) mice. 2 mo after cell transfer, PerC cells were taken from the recipients, stained with indicated antibodies, and analyzed using 10-color flow cytometry. Shown are 5% contour plots of cells gated for exclusion of propidium iodide and expression of the pan-B cell marker CD19. B-1 and B-2 cells were distinguished by differences in their expression of total and allotype-specific IgM and IgD. B-1 cells were further distinguished from B-2 cells by their expression of CD11b (MAC-1) and their low expression of CD21 (data not shown). Percentages indicate frequencies of cells in gate of all PerC (CD19+) B cells.
Figure 4
Lack of sIgM does not affect the T helper response in sIgM−/− mice. Respiratory tract draining mediastinal lymph nodes were isolated from sIgM−/− and sIgM+/+ mice 7 d after infection with influenza A/Mem71. (A) Frequencies of B cell (CD19+) and CD4+ and CD8+CD3+ T cell populations in the mediastinal lymph nodes were assessed by FACS®. (B) Indicated numbers of FACS®-purified CD3+CD4+ T cells from sIgM−/− and sIgM+/+ mice were cultured in the presence of Mem71 virus–pulsed (closed symbols) or nonpulsed (open symbols) irradiated splenic APCs. Incorporation of [3H]thymidine was determined as a measure of T cell proliferation.
Figure 6
Reactivity of B-1 and B-2 cell–derived IgM antibodies before and after infection with influenza virus A/Mem71. Sera from B-1/B-2 allotype-chimeric mice, generated as outlined in the Fig. 5 legend, were taken before and 7 d after infection with influenza virus A/Mem71 to determine the titers of B-1 (Igh-a) and B-2 (Igh-b) cell–derived antibodies able to bind four different influenza virus strains as indicated. Indicated are antiviral titers, determined by ELISA as reciprocal of the serum dilution that resulted in an OD 490 nm reading significantly higher than that of the background. n.s., not significant.
Figure 7
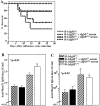
IgM antibodies secreted by both B-1 and B-2 cells are necessary for survival from influenza virus infection and for maximal antiviral IgG2a response. (A) Irradiation chimeras containing B-1 and B-2 cells from indicated sources were constructed as described in Materials and Methods. 2 mo after reconstitution, groups of chimeras (n = 9) were infected with influenza Mem71 at the indicated virus doses, and survival was monitored daily after infection. Similar results were obtained in two independent experiments after infection with 1.6 × 106 PFU per mouse. (B) Chimeras infected with influenza virus Mem71 at 0.8 × 106 PFU per mouse were bled at indicated times after influenza virus infection, and serum levels of virus-specific IgM and IgG2a antibodies were determined by ELISA. Arbitrary units of antivirus Ig per milliliter are shown.
Figure 7
IgM antibodies secreted by both B-1 and B-2 cells are necessary for survival from influenza virus infection and for maximal antiviral IgG2a response. (A) Irradiation chimeras containing B-1 and B-2 cells from indicated sources were constructed as described in Materials and Methods. 2 mo after reconstitution, groups of chimeras (n = 9) were infected with influenza Mem71 at the indicated virus doses, and survival was monitored daily after infection. Similar results were obtained in two independent experiments after infection with 1.6 × 106 PFU per mouse. (B) Chimeras infected with influenza virus Mem71 at 0.8 × 106 PFU per mouse were bled at indicated times after influenza virus infection, and serum levels of virus-specific IgM and IgG2a antibodies were determined by ELISA. Arbitrary units of antivirus Ig per milliliter are shown.
Figure 8
Reconstitution of natural IgM–deficient chimeras results in increased survival and increased antiviral IgG2a serum titers after influenza virus infection. Lethally irradiated recipients were reconstituted with bone marrow from wild-type mice and PerC cells from sIgM−/− mice (B-1 sIgM−/−). Chimeras received daily injections of 0.5 ml of normal serum (sIgM+/+ serum; n = 10) or IgM-deficient serum (sIgM−/− serum; n = 10) immediately before infection and daily for 5 d after infection. Control mice received both bone marrow and PerC cells from sIgM+/+ mice (B-1 sIgM+/+). (A) Survival from infection with influenza A/Mem71 was monitored daily for 28 d. (B) Mem71-binding serum IgM titers were determined by ELISA in groups (n = 9) of noninfected chimeras after 3 d of daily injections with the indicated type of serum or in controls. Arbitrary units of antivirus Ig per milliliter are shown. (C) Mem71-specific IgG2a titers in the sera of indicated groups of irradiation chimeras were determined at day 8 after infection with influenza A/Mem71 by ELISA. Arbitrary units of antivirus Ig per milliliter are shown.
Similar articles
- Role of IgM antibodies versus B cells in influenza virus-specific immunity.
Kopf M, Brombacher F, Bachmann MF. Kopf M, et al. Eur J Immunol. 2002 Aug;32(8):2229-36. doi: 10.1002/1521-4141(200208)32:8<2229::AID-IMMU2229>3.0.CO;2-T. Eur J Immunol. 2002. PMID: 12209635 - Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig.
Topham DJ, Tripp RA, Hamilton-Easton AM, Sarawar SR, Doherty PC. Topham DJ, et al. J Immunol. 1996 Oct 1;157(7):2947-52. J Immunol. 1996. PMID: 8816401 - An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions.
Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. Sangster MY, et al. J Exp Med. 2003 Oct 6;198(7):1011-21. doi: 10.1084/jem.20021745. Epub 2003 Sep 29. J Exp Med. 2003. PMID: 14517272 Free PMC article. - Role of the B-cell response in recovery of mice from primary influenza virus infection.
Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Gerhard W, et al. Immunol Rev. 1997 Oct;159:95-103. doi: 10.1111/j.1600-065x.1997.tb01009.x. Immunol Rev. 1997. PMID: 9416505 Review. - The long elusive IgM Fc receptor, FcμR.
Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, Jones D, Nishida N, Miyawaki T, Bertoli LF, Sanders SK, Honjo K. Kubagawa H, et al. J Clin Immunol. 2014 Jul;34 Suppl 1(0 1):S35-45. doi: 10.1007/s10875-014-0022-7. Epub 2014 May 3. J Clin Immunol. 2014. PMID: 24793544 Free PMC article. Review.
Cited by
- Complement receptors 1 and 2 in murine antibody responses to IgM-complexed and uncomplexed sheep erythrocytes.
Rutemark C, Bergman A, Getahun A, Hallgren J, Henningsson F, Heyman B. Rutemark C, et al. PLoS One. 2012;7(7):e41968. doi: 10.1371/journal.pone.0041968. Epub 2012 Jul 25. PLoS One. 2012. PMID: 22848677 Free PMC article. - A trade-off between natural and acquired antibody production in a reptile: implications for long-term resistance to disease.
Sandmeier FC, Tracy CR, Dupré S, Hunter K. Sandmeier FC, et al. Biol Open. 2012 Nov 15;1(11):1078-82. doi: 10.1242/bio.20122527. Epub 2012 Aug 28. Biol Open. 2012. PMID: 23213387 Free PMC article. - Immunogenicity of replication-deficient vesicular stomatitis virus based rabies vaccine in mice.
Park JE, Shin HJ. Park JE, et al. Vet Q. 2021 Dec;41(1):202-209. doi: 10.1080/01652176.2021.1930277. Vet Q. 2021. PMID: 33985418 Free PMC article. - Natural and induced B-1 cell immunity to infections raises questions of nature versus nurture.
Baumgarth N, Waffarn EE, Nguyen TT. Baumgarth N, et al. Ann N Y Acad Sci. 2015 Dec;1362:188-99. doi: 10.1111/nyas.12804. Epub 2015 Jun 9. Ann N Y Acad Sci. 2015. PMID: 26060895 Free PMC article. - Human B-1 cells take the stage.
Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Rothstein TL, et al. Ann N Y Acad Sci. 2013 May;1285:97-114. doi: 10.1111/nyas.12137. Ann N Y Acad Sci. 2013. PMID: 23692567 Free PMC article. Review.
References
- Coutinho A., Kazatchkine M.D., Avrameas S. Natural autoantibodies. Curr. Opin. Immunol. 1995;7:812–818. - PubMed
- Avrameas S. Natural autoantibodiesfrom ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol. Today. 1991;12:154–159. - PubMed
- Casali P., Schettino E.W. Structure and function of natural antibodies. Curr. Top. Microbiol. Immunol. 1996;210:167–179. - PubMed
- Brandtzaeg P. Overview of the mucosal immune system. Curr. Top. Microbiol. Immunol. 1989;146:13–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases