The gamma-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse - PubMed (original) (raw)
The gamma-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse
M Kneussel et al. Proc Natl Acad Sci U S A. 2000.
Abstract
gamma-Aminobutyric acid type A receptors (GABA(A)Rs) are ligand-gated chloride channels that exist in numerous distinct subunit combinations. At postsynaptic membrane specializations, different GABA(A)R isoforms colocalize with the tubulin-binding protein gephyrin. However, direct interactions of GABA(A)R subunits with gephyrin have not been reported. Recently, the GABA(A)R-associated protein GABARAP was found to bind to the gamma2 subunit of GABA(A)Rs. Here we show that GABARAP interacts with gephyrin in both biochemical assays and transfected cells. Confocal analysis of neurons derived from wild-type and gephyrin-knockout mice revealed that GABARAP is highly enriched in intracellular compartments, but not at gephyrin-positive postsynaptic membrane specializations. Our data indicate that GABARAP-gephyrin interactions are not important for postsynaptic GABA(A)R anchoring but may be implicated in receptor sorting and/or targeting mechanisms. Consistent with this idea, a close homolog of GABARAP, p16, has been found to function as a late-acting intra-Golgi transport factor.
Figures
Figure 1
(A) Sequence alignment of GABARAP, p16, and their homologs from Caenorhabditis elegans, Saccharomyces cerevisiae, and Arabidopsis thaliana. The protein p16, recently described as a bovine late-acting intra-Golgi transport factor (30), shares 57.3% amino acid identity with GABARAP. Identities between GABARAP and its homologs are 78.9% (C. elegans), 54.7% (S. cerevisiae), and 54.2% (A. thaliana), respectively. (B) Phylogenetic tree of GABARAP, p16, and homolog proteins. Note that mammalian p16 is more distant from GABARAP than its homolog from C. elegans.
Figure 2
GABARAP interacts with gephyrin in vitro. GST-GABARAP or GST-GABARAP/36–117 (4 μg each) was bound to glutathione-agarose and incubated with brain homogenate. After washing, bound proteins were eluted with glutathione, separated by SDS/PAGE, and probed with anti-gephyrin. Brain homogenate input (lane 1), GST-GABARAP (lane 2), and GST-GABARAP/36–117 (lane 3) interact with gephyrin, whereas GST alone (lane 4) does not. The GABARAP sequences fused to GST are schematically given (bars) above the gel lanes; putative interaction domains are indicated by arrows.
Figure 3
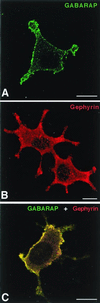
Colocalization of GABARAP and gephyrin upon heterologous expression in PC12 cells. Singly expressed GABARAP protein is located at the plasma membrane (A), whereas singly expressed gephyrin is diffusely distributed with some enrichment at submembranous compartments (B). Coexpression of GABARAP and gephyrin leads to a recruitment of gephyrin to GABARAP-rich loci (C). (Scale bars, 10 μm.)
Figure 4
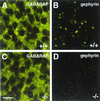
GABARAP immunoreactivity in embryonic day 19.5 spinal cord sections derived from wild-type (+/+) and gephyrin-knockout (−/−) mice. Anti-GABARAP stains punctate structures in spinal cells of wild-type tissue; in addition, a diffuse staining is seen (A). Gephyrin appears in synaptic clusters of wild-type tissue (B, and see ref. 14). In tissue sections derived from gephyrin-deficient mice, GABARAP immunoreactivity is unaltered (C), whereas gephyrin immunoreactivity is lost (D). (Scale bar, 10 μm.)
Figure 5
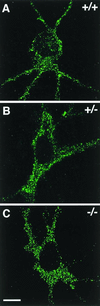
GABARAP staining of cultured cortical neurons derived from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) gephyrin-knockout mice. A punctate distribution of GABARAP (25) is seen throughout the cytoplasm of neurons derived from wild-type (A), heterozygous (B), and homozygous (C) gephyrin-knockout mice. Individual confocal sections are shown. (Scale bar, 10 μm.)
Figure 6
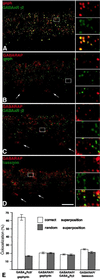
Cofocal micrographs of vertical sections through the inner plexiform layer (IPL) of double-immunostained mouse retinae. Selected areas of the micrographs (frames) are shown at higher magnification, to the right. (A) Gephyrin (red) and the γ2 subunit of the GABAAR (green) are aggregated in synaptic hot spots, which are often colocalized. (B) GABARAP (red) shows diffuse, punctate distribution in the IPL but is not clustered with gephyrin (green) in synaptic hot spots. The white arrows point to the cell bodies of ganglion cells and show the expression of GABARAP in the cytoplasm. (C) GABARAP (red) and the γ2 subunit of the GABAAR (green) appear not to be aggregated within the same hot spots. (D) The presynaptic cytomatrix protein bassoon (green), which is clustered at both excitatory and inhibitory synapses, is not colocalized with GABARAP. (Scale bar, 10 μm.) (E) Quantifications of the colocalizations at their correct superpositions and at random superpositions. Only in the case of the γ2 subunit of the GABAAR and gephyrin was a significant colocalization of puncta observed.
Similar articles
- GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton.
Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. Wang H, et al. Nature. 1999 Jan 7;397(6714):69-72. doi: 10.1038/16264. Nature. 1999. PMID: 9892355 - The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors.
Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. Kittler JT, et al. Mol Cell Neurosci. 2001 Jul;18(1):13-25. doi: 10.1006/mcne.2001.1005. Mol Cell Neurosci. 2001. PMID: 11461150 - Dynamic regulation of GABA(A) receptors at synaptic sites.
Kneussel M. Kneussel M. Brain Res Brain Res Rev. 2002 Jun;39(1):74-83. doi: 10.1016/s0165-0173(02)00159-5. Brain Res Brain Res Rev. 2002. PMID: 12086709 Review. - Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells.
Panzanelli P, Gunn BG, Schlatter MC, Benke D, Tyagarajan SK, Scheiffele P, Belelli D, Lambert JJ, Rudolph U, Fritschy JM. Panzanelli P, et al. J Physiol. 2011 Oct 15;589(Pt 20):4959-80. doi: 10.1113/jphysiol.2011.216028. Epub 2011 Aug 8. J Physiol. 2011. PMID: 21825022 Free PMC article. - Intracellular trafficking of GABA(A) receptors.
Barnes EM Jr. Barnes EM Jr. Life Sci. 2000 Feb 11;66(12):1063-70. doi: 10.1016/s0024-3205(99)00469-5. Life Sci. 2000. PMID: 10737356 Review.
Cited by
- Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts.
Marchand S, Devillers-Thiéry A, Pons S, Changeux JP, Cartaud J. Marchand S, et al. J Neurosci. 2002 Oct 15;22(20):8891-901. doi: 10.1523/JNEUROSCI.22-20-08891.2002. J Neurosci. 2002. PMID: 12388596 Free PMC article. - 1H, 13C and '5N resonance assignments of GABARAP, GABAA receptor associated protein.
Kouno T, Miura K, Kanematsu T, Shirakawa M, Hirata M, Kawano K. Kouno T, et al. J Biomol NMR. 2002 Jan;22(1):97-8. doi: 10.1023/a:1013884402033. J Biomol NMR. 2002. PMID: 11885988 No abstract available. - Deciphering the structural framework of glycine receptor anchoring by gephyrin.
Kim EY, Schrader N, Smolinsky B, Bedet C, Vannier C, Schwarz G, Schindelin H. Kim EY, et al. EMBO J. 2006 Mar 22;25(6):1385-95. doi: 10.1038/sj.emboj.7601029. Epub 2006 Mar 2. EMBO J. 2006. PMID: 16511563 Free PMC article. - Complex role of collybistin and gephyrin in GABAA receptor clustering.
Saiepour L, Fuchs C, Patrizi A, Sassoè-Pognetto M, Harvey RJ, Harvey K. Saiepour L, et al. J Biol Chem. 2010 Sep 17;285(38):29623-31. doi: 10.1074/jbc.M110.121368. Epub 2010 Jul 9. J Biol Chem. 2010. PMID: 20622020 Free PMC article. - The trafficking protein GABARAP binds to and enhances plasma membrane expression and function of the angiotensin II type 1 receptor.
Cook JL, Re RN, deHaro DL, Abadie JM, Peters M, Alam J. Cook JL, et al. Circ Res. 2008 Jun 20;102(12):1539-47. doi: 10.1161/CIRCRESAHA.108.176594. Epub 2008 May 22. Circ Res. 2008. PMID: 18497328 Free PMC article.
References
- Sheng M. Neuron. 1996;17:575–578. - PubMed
- Craven S E, Bredt D S. Cell. 1998;93:495–498. - PubMed
- Schmitt B, Knaus P, Becker C M, Betz H. Biochemistry. 1987;26:805–811. - PubMed
- Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J, Betz H. Neuron. 1992;8:1161–1170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases