Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus-induced cell-cell fusion but not virus-cell fusion - PubMed (original) (raw)
Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus-induced cell-cell fusion but not virus-cell fusion
E Schmid et al. J Virol. 2000 Aug.
Abstract
Canine distemper virus (CDV) causes a life-threatening disease in several carnivores including domestic dogs. Recently, we identified a molecule, CD9, a member of the tetraspan transmembrane protein family, which facilitates, and antibodies to which inhibit, the infection of tissue culture cells with CDV (strain Onderstepoort). Here we describe that an anti-CD9 monoclonal antibody (MAb K41) did not interfere with binding of CDV to cells and uptake of virus. In addition, in single-step growth experiments, MAb K41 did not induce differences in the levels of viral mRNA and proteins. However, the virus release of syncytium-forming strains of CDV, the virus-induced cell-cell fusion in lytically infected cultures, and the cell-cell fusion of uninfected with persistently CDV-infected HeLa cells were strongly inhibited by MAb K41. These data indicate that anti-CD9 antibodies selectively block virus-induced cell-cell fusion, whereas virus-cell fusion is not affected.
Figures
FIG. 1
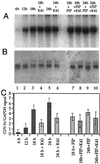
Anti-CD9 antibodies do not inhibit the binding to and the uptake of CDV by cells. CDV strain OND-SP (MOI = 10) was bound to Vero cells at 4°C, and the bound virus was detected by flow cytometry with a polyclonal dog hyperimmune serum (A). Bound virus shifted the signal from background (Vero) to high values of mean fluorescence intensity (Vero + CDV). Preincubation with MAb K41 (50 μg/ml) did not block the binding of virus and gives similar signals as virus alone (Vero + K41 + CDV). The mean values of the median fluorescence intensities of three binding experiments in the presence and absence of MAb K41 are shown in panel B. To measure the uptake of CDV by RT-PCR (C), Vero cells were infected with decreasing MOIs of CDV (1, 0.5, 0.1, 0.05, and 0.01) in the absence and presence of MAb K41 (lanes 1 to 5 and 6 to 10, respectively). The negative controls (lane 11 and 17) were RNA from uninfected cells; the positive control (lane 12) was RNA from 48-h CDV-infected Vero cells (MOI = 0.1). As a control, Vero cells were incubated with CDV at MOIs 3 and 1 at 4°C (lanes 13 and 14) and at 37°C (lanes 15 and 16) for 2 h and washed with the acidic buffer and PBS. Reverse transcription was primed with the primer specific for the CDV genome (forward primer). PCR with the F/H primers amplifies a 1,027-bp fragment spanning parts of the F and H genes of CDV. The RNA used for the RT-PCRs is shown in panel D.
FIG. 2
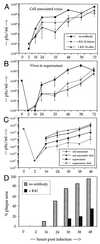
Titration of cell-associated and released virus from CDV-infected cells. (A and B) Vero cells were mock treated or treated with MAb K41 for 1 h before or 1 h after infection with OND-SP (as indicated; MOI = 0.01). After treatment with K41 (12 μg/ml), the cells were washed to remove unbound antibodies and incubated for the indicated times. Cell-associated virus released by one cycle of freezing-thawing (A) and supernatant (B) was titrated on Vero cells (n = 3). (C and D) Vero cells were infected at an MOI of 3.0 in the presence or absence of MAb K41, and cell-associated and cell-free virus was titrated (C). The percentage of the culture area in plaques was calculated using an image assistant program by computer, considering the area of syncytia containing three or more nuclei (D).
FIG. 3
No effect of MAb K41 on CDV N-specific mRNA levels. Vero cells were infected with OND-SP (MOI = 0.5), the inoculum was removed, and MAb K41 (12 μg/ml) was added to the cultures. RNA was harvested after 0, 6, and 12 hpi and blotted on Hybond-N filters. The filters were hybridized with 32P-labeled probes specific for CDV N (A) and GAPDH (B). Two experiments are shown (lanes 1 to 4 and lanes 5 to 8). Mean values of the N/GAPDH signal ratio are given in panel C.
FIG. 4
MAb K41 does not inhibit viral protein synthesis. Vero cells were infected with OND-SP (MOI = 3) for 6, 12, 18, 24, and 30 h in the presence and absence of K41 (12 μg/ml), as indicated. Cells were labeled for 5 h with [35S]methionine and [35S]cysteine and subsequently immunoprecipitated with a polyclonal anti-CDV antiserum (lanes 1 to 14) or MAb K41 (lane 15) and protein A-Sepharose beads. In lanes 4, 6, 8, 10, and 12, MAb K41 was added after the infection (at 1 hpi) and also during the incubation with [35S]methionine and [35S]cysteine. The viral proteins P, H, N, F1 (lower band below actin), M, and F2 were detected as published elsewhere (33). Superimposed on the F1 band, a background signal is present in all lanes. MAb K41 present in the cultures led to a visible CD9 band (lanes 4, 6, 8, 10, 12, and 14), similar to the result obtained when it was used for precipitation (lane 15).
FIG. 5
Determination of the effect of MAb K41 on the extent of cell-cell fusion. Uninfected and persistently CDV-infected HeLa cells were mixed in the absence of antibodies (A, D, G, and J) or in the presence of MAb K41 (B, E, H, and K) or anti-CDV-H MAb (C, F, I, and L). Coverslips were processed for immunofluorescence after 1, 10, and 22 h. (A to F) For early time points, uninfected cells were labeled with rhodamine R18 (red), and infected cells were labeled with calcein (green). In the case of cell-cell fusion, the color of growing syncytia changes from green to orange (D, arrows). In the presence of K41, only a few small syncytia are observed after 10 h (E, arrows). In the presence of anti-CDV H, cell-cell fusion is completely inhibited (F). (G to L) To visualize larger syncytia at later time points, the nuclei of uninfected cells were labeled with Hoechst H33258 (blue), and infected cells were labeled with rhodamine R18 plus calcein (yellow). Large syncytia are formed in the absence of antibodies and turn red (J). In the presence of K41, small syncytia develop (K), whereas in the presence of anti-CDV H, only single persistently infected cells are present and cell-cell fusion is completely blocked (L).
FIG. 6
Effect of MAb K41 and FIP on CDV N mRNA levels under conditions allowing virus spread by cell-cell fusion. Vero cells were infected with CDV strain OND-SP at a low MOI of 0.1. MAb K41 (12 μg/ml) alone, FIP (200 μg/ml) alone, or combinations of the two were added to the cultures after 12 h as indicated. RNA was harvested at 0, 6, 12, 18, and 24 hpi, blotted on Hybond-N filters, and hybridized to 32P-labeled probes for CDV N (A) and GAPDH (B). Mean values of the N/GAPDH signal ratio are given in panel C.
FIG. 7
No effect of MAb K41 on infection of Vero cells with CDV strain RB. Vero cells were infected with the non-syncytium-forming CDV strain RB (MOI = 0.01) for 1 h prior to addition of anti-CD9 MAb K41 (12 μg/ml) and analyzed by flow cytometry after 0, 3, 4, 5, 6, and 7 dpi. The CDV N-specific signal in the absence (full line) and presence (dashed line) of K41 is shown.
Similar articles
- CD9, a tetraspan transmembrane protein, renders cells susceptible to canine distemper virus.
Löffler S, Lottspeich F, Lanza F, Azorsa DO, ter Meulen V, Schneider-Schaulies J. Löffler S, et al. J Virol. 1997 Jan;71(1):42-9. doi: 10.1128/JVI.71.1.42-49.1997. J Virol. 1997. PMID: 8985321 Free PMC article. - CD9-dependent regulation of Canine distemper virus-induced cell-cell fusion segregates with the extracellular domain of the haemagglutinin.
Singethan K, Topfstedt E, Schubert S, Duprex WP, Rima BK, Schneider-Schaulies J. Singethan K, et al. J Gen Virol. 2006 Jun;87(Pt 6):1635-1642. doi: 10.1099/vir.0.81629-0. J Gen Virol. 2006. PMID: 16690928 - SLAM- and nectin-4-independent noncytolytic spread of canine distemper virus in astrocytes.
Alves L, Khosravi M, Avila M, Ader-Ebert N, Bringolf F, Zurbriggen A, Vandevelde M, Plattet P. Alves L, et al. J Virol. 2015 May;89(10):5724-33. doi: 10.1128/JVI.00004-15. Epub 2015 Mar 18. J Virol. 2015. PMID: 25787275 Free PMC article. - Tropism and molecular pathogenesis of canine distemper virus.
Rendon-Marin S, da Fontoura Budaszewski R, Canal CW, Ruiz-Saenz J. Rendon-Marin S, et al. Virol J. 2019 Mar 7;16(1):30. doi: 10.1186/s12985-019-1136-6. Virol J. 2019. PMID: 30845967 Free PMC article. Review. - Canine distemper virus and multiple sclerosis.
Hodge MJ, Wolfson C. Hodge MJ, et al. Neurology. 1997 Aug;49(2 Suppl 2):S62-9. doi: 10.1212/wnl.49.2_suppl_2.s62. Neurology. 1997. PMID: 9270694 Review. No abstract available.
Cited by
- Inhibition of HIV-1 replication by nanobodies targeting tetraspanin CD9.
Umotoy JC, Kroon PZ, Man S, van Dort KA, Atabey T, Schriek AI, Dekkers G, Herrera-Carrillo E, Geijtenbeek TBH, Heukers R, Kootstra NA, van Gils MJ, de Taeye SW. Umotoy JC, et al. iScience. 2024 Sep 13;27(10):110958. doi: 10.1016/j.isci.2024.110958. eCollection 2024 Oct 18. iScience. 2024. PMID: 39391729 Free PMC article. - Cellular proteins in influenza virus particles.
Shaw ML, Stone KL, Colangelo CM, Gulcicek EE, Palese P. Shaw ML, et al. PLoS Pathog. 2008 Jun 6;4(6):e1000085. doi: 10.1371/journal.ppat.1000085. PLoS Pathog. 2008. PMID: 18535660 Free PMC article. - Tetraspanin CD9 determines invasiveness and tumorigenicity of human breast cancer cells.
Rappa G, Green TM, Karbanová J, Corbeil D, Lorico A. Rappa G, et al. Oncotarget. 2015 Apr 10;6(10):7970-91. doi: 10.18632/oncotarget.3419. Oncotarget. 2015. PMID: 25762645 Free PMC article. - Tetraspanins are involved in Burkholderia pseudomallei-induced cell-to-cell fusion of phagocytic and non-phagocytic cells.
Sangsri T, Saiprom N, Tubsuwan A, Monk P, Partridge LJ, Chantratita N. Sangsri T, et al. Sci Rep. 2020 Oct 21;10(1):17972. doi: 10.1038/s41598-020-74737-y. Sci Rep. 2020. PMID: 33087788 Free PMC article. - The many mechanisms of viral membrane fusion proteins.
Earp LJ, Delos SE, Park HE, White JM. Earp LJ, et al. Curr Top Microbiol Immunol. 2005;285:25-66. doi: 10.1007/3-540-26764-6_2. Curr Top Microbiol Immunol. 2005. PMID: 15609500 Free PMC article. Review.
References
- Allen I V, McQuaid S, McMahon J, Kirk J, McConnel R. The significance of measles virus antigen and genome distribution in the CNS in SSPE for mechanisms of viral spread and demyelination. J Neuropathol Exp Neurol. 1996;55:471–480. - PubMed
- Appel M J G, Gillespie J H. Canine distemper virus. Virol Monogr. 1972;11:1–96.
- Barrett T, Visser I K G, Mamaev L, Goatley L, van Bressem M-F, Osterhaus A D M E. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology. 1993;193:1010–1012. - PubMed
- Benoit P, Gross M S, Frachet P, Frezal J, Uzan G, Boucheix C, Nguyen V C. Assignment of the human CD9 gene to chromosome 12 (region P13) by use of human specific DNA probes. Hum Genet. 1991;86:268–272. - PubMed
- Boucheix C, Benoit P, Frachet P, Billard M, Worthington R E, Gagnon J, Uzan G. Molecular cloning of the CD9 antigen. A new family of cell surface proteins. J Biol Chem. 1991;266:117–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources