A functional link between dynamin and the actin cytoskeleton at podosomes - PubMed (original) (raw)
A functional link between dynamin and the actin cytoskeleton at podosomes
G C Ochoa et al. J Cell Biol. 2000.
Abstract
Cell transformation by Rous sarcoma virus results in a dramatic change of adhesion structures with the substratum. Adhesion plaques are replaced by dot-like attachment sites called podosomes. Podosomes are also found constitutively in motile nontransformed cells such as leukocytes, macrophages, and osteoclasts. They are represented by columnar arrays of actin which are perpendicular to the substratum and contain tubular invaginations of the plasma membrane. Given the similarity of these tubules to those generated by dynamin around a variety of membrane templates, we investigated whether dynamin is present at podosomes. Immunoreactivities for dynamin 2 and for the dynamin 2-binding protein endophilin 2 (SH3P8) were detected at podosomes of transformed cells and osteoclasts. Furthermore, GFP wild-type dynamin 2aa was targeted to podosomes. As shown by fluorescence recovery after photobleaching, GFP-dynamin 2aa and GFP-actin had a very rapid and similar turnover at podosomes. Expression of the GFP-dynamin 2aa(G273D) abolished podosomes while GFP-dynamin(K44A) was targeted to podosomes but delayed actin turnover. These data demonstrate a functional link between a member of the dynamin family and actin at attachment sites between cells and the substratum.
Figures
Figure 1
Colocalization of dynamin 2 and endophilin 2 with a variety of podosomal markers and with filamentous actin in podosomes rosettes of RSV-transformed BHK21 cells. Serum-starved cells were reacted by immunofluorescence for the proteins indicated and counterstained with phalloidin. Dynamin was labeled with two different antibodies, Dyn2 and Hudy-1, and only Dyn 2 labels podosomes. Bar, 14 μM.
Figure 2
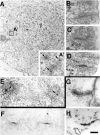
Comparative electron micrographs of tubular membrane invaginations surrounded by an actin sheath at podosomes and in synaptic membrane preparations incubated in cell-free conditions. (A) RVS-transformed BHK21 cell cut parallel to the substratum demonstrating an actin-rich ring (dashed lines) which excludes other organelles and which results from the rosette-like apposition of individual podosomes. The presence of a cross-sectioned tubule in the core of each podosomes (arrows) is shown in A′, which represents a high magnification of the regions enclosed by a square. (B–D) High power views of three podosomes from sections of RVS-transformed BHK21 cells cut perpendicular to the substratum and demonstrating the presence of tubular plasma membrane invaginations. (E) High magnification of two podosomes from the peripheral region of a mouse osteoclast grown on a coverslip and cut parallel to the substratum. Arrows point to cross-sectioned tubules. (F–H) Dynamin-coated tubules from synaptic membranes incubated with brain cytosol, ATP, and GTPγS. G and H show immunolabeling for dynamin and actin, respectively. Note the similarity of these structures to podosomes. Bar: (A) 0.6 nm; (A′ and B–H) 200 nM.
Figure 3
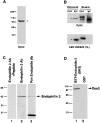
The Dyn2 antibody recognizes selectively dynamin in RSV-transformed BHK21 cell extracts and dynamin 2 specifically interacts with endophilin 2. (A) Western blot of a detergent extract of RSV-transformed BHK21 cells with the Dyn2 antibody. (B) Triton X-100 extracts of RSV-transformed BHK21 cells were immunoprecipitated with Dyn2 antibodies or control IgGs and the immunoprecipitates were reacted by Western blotting with either the Dyn2 antibody (top) or a commercial anti-dynamin antibody (#D25520 from Transduction Laboratories) which recognizes both dynamin 1 and 2. Note that the Dyn2 antibody depletes dynamin immunoreactivity (arrows) from the extract. The slower mobility of dynamin 2 in the bound material was consistently observed and may reflect the absence of Triton X-100 in the load. (C) Extracts of RSV-transformed BHK21 cells extracts were reacted by Western blotting with an endophilin 2-specific antibody in the presence or absence of the peptide used as the immunogen (lanes 1 and 2) or with a pan-endophilin antibody (lane 3). (D) A Triton X-100 extract of RSV-transformed BHK21 cells was incubated with either a GST fusion protein of the SH3 domain of endophilin 2 (lane 1) or GST alone (lane 2). The bound material was then reacted by Western blotting with the Dyn2 antibody.
Figure 6
Immunolocalization of dynamin 2 and endophilin 2 in the podosomes of osteoclasts. (A–D) Mouse osteoclasts were immunostained for dynamin 2 and counterstained for actin with phalloidin. All three proteins colocalize in the peripheral sealing zone known to be enriched in podosomes. (E and F) The cDNA encoding GFP-dynamin 2aa was microinjected in the nuclei of the osteoclast. After 6 h, cells were fixed and counterstained for actin with phalloidin. Bar, 42 μM.
Figure 4
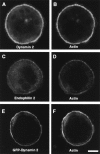
Colocalization of dynamin 2 immunoreactivity and actin in RSV-transformed and nontransformed BHK21 cells. (A–D) RSV-transformed and nontransformed BHK21 cells were stained by immunofluorescence for dynamin 2 with the Dyn2 antibody and for actin with phalloidin. In both cells a partial colocalization of dynamin 2 with actin is observed even though the podosomes rosette is only visible in transformed cells.
Figure 5
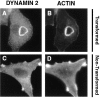
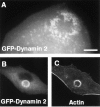
Targeting of transfected dynamin 2aa to podosomes of RSV-transformed BHK21 cells. (A) A RSV-transformed BHK21 cell was transfected with GFP-dynamin 2aa and examined by a CCD camera 12 h after transfected. In this living cell GFP fluorescence reveals individual podosomes. Most, but not all of them, are clustered at the rosette. (B and C) RSV-transformed BHK21 were transformed with GFP-dynamin 2aa, fixed, and then counterstained for actin with phalloidin. Bar: (A) 7 μM; (B and C) 14 μM.
Figure 7
Treatment of osteoclasts with the calcineurin inhibitor cyclosporin A has disruptive affect on podosomes. Phalloidin staining revealing the progressive disruption of podosomes in osteoclasts which were treated with 20 μM cyclosporin A for either 45 (B) or 90 min (C), as compared with a nontreated osteoclast (A). Bar, 70 μM.
Figure 8
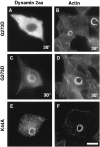
Expression of dynamin 2aaG273D and dynamin 2aaK44A in transformed BHK21 cells. RSV-transformed BHK21 cells were transiently transfected with GFP-dynamin 2aaG273D or with GFP-dynamin 2aaK44A as indicated. After transfection cells were kept at either 30°C or 38°C as also indicated. In the cells kept at 38°C no podosomes are present. GFP-dynamin 2aaK44A is targeted to the podosome rosettes. All cells were counterstained for actin with phalloidin. Bar, 14 μM.
Figure 10
Schematic representation of podosomes and of potential roles of actin in endocytosis. (A) Podosomes (Nitsch et al. 1989), which are represented by columnar arrays of actin enclosing a very narrow tubular invagination of the plasma membrane, have a structure very similar to that previously described for yeast actin patches (inset; Mulholland et al. 1994). The drawing on the right depicts the hypothesis that membrane may flow in the tubular invaginations and that endocytic vesicles may pinch off from their ends. (B) Drawing illustrating the possibility that a cytoskeletal scaffold at the neck of clathrin coated pits may resemble the scaffold surrounding the tubular invagination of podosomes. This scaffold could assist the fission reaction by twisting the neck (left) or by pushing away form the plasma membrane (right). The residual scaffold at the point of fission may drive the formation of actin comets.
Figure 9
Fluorescence recovery after photobleaching (FRAP) of GFP-actin and GFP-dynamin. RSV-transformed BHK21 cells were transiently transfected with GFP-actin or GFP-dynamin, then bleached for 20 s, and allowed to recover. (A) Micrographs of GFP-actin appearance before bleaching, immediately after bleach, and 1 min post-bleach. (B) Time course of GFP-actin fluorescence in the section of the rosette subjected to photobleaching (closed diamonds) and in a nonbleached region (open diamonds). Fluorescence fully recovered after 60 s with a rate constant of 1.89 ± 1.41 min−1 (r 2 = 0.881 ± 0.99). (C) Same as B in the presence of jasplakinolide (1 μM). No recovery occurs in the presence of this drug. (D–F) GFP-dynamin 2aa recovered at a rate very similar to that of GFP-actin, while GFP-dynamin 2aaK44A recovered at a much slower rate. E shows the rate constants for GFP-dynamin (k = 1.89 ± 0.236 min−1, r 2 = 0.845 ± 0.01) and GFP-dynamin 2aaK44A (k = 0.55 ± 0.57 min−1, r 2 = 0.969 ± 01) and illustrates the statistically significant difference between the two rates. Bar, 14 μM.
Similar articles
- Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture.
Akisaka T, Yoshida H, Suzuki R, Takama K. Akisaka T, et al. Cell Tissue Res. 2008 Mar;331(3):625-41. doi: 10.1007/s00441-007-0552-x. Epub 2007 Dec 18. Cell Tissue Res. 2008. PMID: 18087726 - Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity.
Bruzzaniti A, Neff L, Sanjay A, Horne WC, De Camilli P, Baron R. Bruzzaniti A, et al. Mol Biol Cell. 2005 Jul;16(7):3301-13. doi: 10.1091/mbc.e04-12-1117. Epub 2005 May 4. Mol Biol Cell. 2005. PMID: 15872089 Free PMC article. - Essential function of dynamin in the invasive properties and actin architecture of v-Src induced podosomes/invadosomes.
Destaing O, Ferguson SM, Grichine A, Oddou C, De Camilli P, Albiges-Rizo C, Baron R. Destaing O, et al. PLoS One. 2013 Dec 9;8(12):e77956. doi: 10.1371/journal.pone.0077956. eCollection 2013. PLoS One. 2013. PMID: 24348990 Free PMC article. - The role of dynamin in the assembly and function of podosomes and invadopodia.
McNiven MA, Baldassarre M, Buccione R. McNiven MA, et al. Front Biosci. 2004 May 1;9:1944-53. doi: 10.2741/1348. Front Biosci. 2004. PMID: 14977600 Review. - Podosome and sealing zone: specificity of the osteoclast model.
Jurdic P, Saltel F, Chabadel A, Destaing O. Jurdic P, et al. Eur J Cell Biol. 2006 Apr;85(3-4):195-202. doi: 10.1016/j.ejcb.2005.09.008. Epub 2005 Oct 24. Eur J Cell Biol. 2006. PMID: 16546562 Review.
Cited by
- Temporal changes in plasma membrane lipid content induce endocytosis to regulate developmental epithelial-to-mesenchymal transition.
Piacentino ML, Hutchins EJ, Andrews CJ, Bronner ME. Piacentino ML, et al. Proc Natl Acad Sci U S A. 2022 Dec 20;119(51):e2212879119. doi: 10.1073/pnas.2212879119. Epub 2022 Dec 12. Proc Natl Acad Sci U S A. 2022. PMID: 36508654 Free PMC article. - The Lipid-Binding Defective Dynamin 2 Mutant in Charcot-Marie-Tooth Disease Impairs Proper Actin Bundling and Actin Organization in Glomerular Podocytes.
Hamasaki E, Wakita N, Yasuoka H, Nagaoka H, Morita M, Takashima E, Uchihashi T, Takeda T, Abe T, Lee JW, Iimura T, Saleem MA, Ogo N, Asai A, Narita A, Takei K, Yamada H. Hamasaki E, et al. Front Cell Dev Biol. 2022 May 10;10:884509. doi: 10.3389/fcell.2022.884509. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35620056 Free PMC article. - The BAR domain superfamily: membrane-molding macromolecules.
Frost A, Unger VM, De Camilli P. Frost A, et al. Cell. 2009 Apr 17;137(2):191-6. doi: 10.1016/j.cell.2009.04.010. Cell. 2009. PMID: 19379681 Free PMC article. - Tks5 and Dynamin-2 enhance actin bundle rigidity in invadosomes to promote myoblast fusion.
Chuang MC, Lin SS, Ohniwa RL, Lee GH, Su YA, Chang YC, Tang MJ, Liu YW. Chuang MC, et al. J Cell Biol. 2019 May 6;218(5):1670-1685. doi: 10.1083/jcb.201809161. Epub 2019 Mar 20. J Cell Biol. 2019. PMID: 30894403 Free PMC article. - A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique.
Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T. Fujita A, et al. Proc Natl Acad Sci U S A. 2009 Jun 9;106(23):9256-61. doi: 10.1073/pnas.0900216106. Epub 2009 May 22. Proc Natl Acad Sci U S A. 2009. PMID: 19470488 Free PMC article.
References
- Adams A.E., Botstein D., Drubin D.G. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991;354:404–408. - PubMed
- Ahn S., Maudsley S., Luttrell L.M., Lefkowitz R.J., Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 1999;274:1185–1188. - PubMed
- Ali N.N., Boyde A., Jones S.J. Motility and resorptionosteoclastic activity in vitro . Anat. Embryol. (Ber) 1984;170:51–56. - PubMed
- Babb S.G., Matsudaira P., Sato M., Correia I., Lim S.S. Fimbrin in podosomes of monocyte-derived osteoclasts. Cell Motil. Cytoskelet. 1997;37:308–325. - PubMed
- Balguerie A., Sivadon P., Bonneu M., Aigle M. Rvs167p, the budding yeast homolog of amphiphysin, colocalizes with actin patches. J. Cell Sci. 1999;112:2529–2537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR042927/AR/NIAMS NIH HHS/United States
- AR42927/AR/NIAMS NIH HHS/United States
- P01 CA046128/CA/NCI NIH HHS/United States
- CA46128/CA/NCI NIH HHS/United States
- R01 NS036251/NS/NINDS NIH HHS/United States
- NS36251/NS/NINDS NIH HHS/United States
- R37 NS036251/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials