Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy - PubMed (original) (raw)
Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy
D E Syroid et al. J Neurosci. 2000.
Abstract
Schwann cells express the low-affinity neurotrophin receptor (p75), but no role for either the neurotrophins or their cognate receptors in Schwann cell development has been established. We have found that Schwann cells isolated from postnatal day 1 (P1) or P2 mice that were p75-deficient exhibited potentiated survival compared to wild-type cells after growth factor and serum withdrawal. There was, however, no disparity in the survival of p75-deficient and wild-type Schwann cells isolated at embryonic day 15, suggesting that the death-inducing effects of p75 are developmentally regulated. A comparable degree of cell death was also observed in the sciatic nerves of both wild-type and p75-deficient mice at P1. However, 24 hr after axotomy, there was a 13-fold increase in the percentage of apoptotic nuclei in the distal nerve stumps of the transected sciatic nerves of neonatal wild-type but not p75-deficient mice. The expression of both the p75 and nerve growth factor (NGF) genes was upregulated after axotomy in neonatal wild-type nerves. Collectively, these results suggest that NGF-mediated activation of p75 is likely to be an important mediator of Schwann cell apoptosis in the context of peripheral nerve injury.
Figures
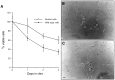
Fig. 1.
Postnatal Schwann cells isolated from p75-deficient mice exhibit a survival advantage in vitro, in comparison to wild-type cells, after growth factor withdrawal. Shown in A is a graphical representation of survival of postnatal cells using an MTT assay, from a representative experiment of three independent assessments, with five separate wells assessed daily over a 3 d period. Shown in B and_C_ are photomicrographs of cultures of postnatal wild-type (B) and p75-deficient (C) cells grown in serum-free conditions for 48 hr demonstrating a large number of crenated wild-type cells (arrowheads), whereas the majority of p75-deficient cells maintain a bipolar morphology. Scale bar, 10 μm.
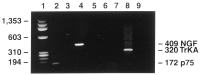
Fig. 2.
The p75 and NGF genes are expressed by postnatal wild-type Schwann cells after growth factor withdrawal, but the trkA gene is expressed at barely detectable levels. cDNA samples generated from purified wild-type Schwann cells were subjected to PCR. The expected 172 bp p75 (lane 2) and 409 bp NGF (lane 4) fragments were amplified from the sample. The expected 320 bp (lane 6) trkA fragment was also amplified, although at a very low level. This contrasted with the robust amplification of the expected 320 bp trkA fragment in a cDNA sample derived from PC12 cells (lane 8). Negative controls were obtained by subjecting reagents to PCR reaction conditions without template cDNA (p75 primers, _lane 3;_NGF primers, lane 5; trkA primers, lane 7), and there was no amplification of the relevant bands. Lane 1 represents the ΦX174_Hae_III DNA ladder.
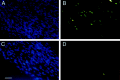
Fig. 3.
Schwann cell apoptosis is upregulated in the axotomized nerves of P1 wild-type mice in comparison to that exhibited in the axotomized nerves of p75-deficient mice. The sciatic nerves of P1 mice were axotomized, and the distal stumps were harvested 24 hr later. The sectioned nerves were assessed using DAPI staining to identify nuclei (A, C) and by TUNEL (B, D). A significantly increased number and percentage of condensed and TUNEL-positive nuclei were present in the wild-type nerves (A, B) in comparison to the number observed in nerves isolated from p75-deficient mice (C, D). Scale bar, 10 μm.
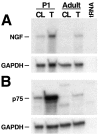
Fig. 4.
Expression of NGF and p75 mRNA is upregulated in rat sciatic nerve during Wallerian degeneration. Unilateral transections of P1 and adult rat sciatic nerves were performed, and ribonuclease protection analyses were performed using total RNA (1 μg per hybridization reaction) isolated from both the intact contralateral nerve (CL) and the transected distal nerve stump (T), 24 hr after transection, or using 10 μg of tRNA as a negative control, as indicated. NGF expression (A) and p75 expression (B) in the degenerating neonatal nerve is upregulated by ∼3-fold and 14-fold, respectively, relative to that in the contralateral control nerve. NGF and p75 expression is also upregulated in degenerating adult nerves, but the level of expression only reaches that found in the intact P1 nerve. The major 484 bp protected NGF RNA fragment and the 452 bp protected p75 RNA fragment are indicated. Autoradiographic exposure was for 66 hr. The bottom panels in A and_B_ show the relative GAPDH expression from corresponding cohybridization reactions using the GAPDH RNA probe. Autoradiographic exposure was for 20 hr.
Similar articles
- Schwann cell apoptosis in the postnatal axotomized sciatic nerve is mediated via NGF through the low-affinity neurotrophin receptor.
Petratos S, Butzkueven H, Shipham K, Cooper H, Bucci T, Reid K, Lopes E, Emery B, Cheema SS, Kilpatrick TJ. Petratos S, et al. J Neuropathol Exp Neurol. 2003 Apr;62(4):398-411. doi: 10.1093/jnen/62.4.398. J Neuropathol Exp Neurol. 2003. PMID: 12722832 - Merlin status regulates p75(NTR) expression and apoptotic signaling in Schwann cells following nerve injury.
Ahmad I, Fernando A, Gurgel R, Jason Clark J, Xu L, Hansen MR. Ahmad I, et al. Neurobiol Dis. 2015 Oct;82:114-122. doi: 10.1016/j.nbd.2015.05.021. Epub 2015 Jun 6. Neurobiol Dis. 2015. PMID: 26057084 Free PMC article. - Improved survival of injured sciatic nerve Schwann cells in mice lacking the p75 receptor.
Ferri CC, Bisby MA. Ferri CC, et al. Neurosci Lett. 1999 Sep 17;272(3):191-4. doi: 10.1016/s0304-3940(99)00618-7. Neurosci Lett. 1999. PMID: 10505613 - Nerve growth factor signaling through p75 induces apoptosis in Schwann cells via a Bcl-2-independent pathway.
Soilu-Hänninen M, Ekert P, Bucci T, Syroid D, Bartlett PF, Kilpatrick TJ. Soilu-Hänninen M, et al. J Neurosci. 1999 Jun 15;19(12):4828-38. doi: 10.1523/JNEUROSCI.19-12-04828.1999. J Neurosci. 1999. PMID: 10366617 Free PMC article. - Nerve growth factor signaling of p75 induces differentiation and ceramide-mediated apoptosis in Schwann cells cultured from degenerating nerves.
Hirata H, Hibasami H, Yoshida T, Ogawa M, Matsumoto M, Morita A, Uchida A. Hirata H, et al. Glia. 2001 Dec;36(3):245-58. doi: 10.1002/glia.1113. Glia. 2001. PMID: 11746763
Cited by
- Hearing loss and vestibular schwannoma: new insights into Schwann cells implication.
Mohamed T, Melfi V, Colciago A, Magnaghi V. Mohamed T, et al. Cell Death Dis. 2023 Sep 23;14(9):629. doi: 10.1038/s41419-023-06141-z. Cell Death Dis. 2023. PMID: 37741837 Free PMC article. Review. - Thrombin-Induced Microglia Activation Modulated through Aryl Hydrocarbon Receptors.
Sheu ML, Pan LY, Yang CN, Sheehan J, Pan LY, You WC, Wang CC, Pan HC. Sheu ML, et al. Int J Mol Sci. 2023 Jul 13;24(14):11416. doi: 10.3390/ijms241411416. Int J Mol Sci. 2023. PMID: 37511175 Free PMC article. - Retrograde apoptotic signaling by the p75 neurotrophin receptor.
Pathak A, Carter BD. Pathak A, et al. Neuronal Signal. 2017 Feb 24;1(1):NS20160007. doi: 10.1042/NS20160007. eCollection 2017 Feb. Neuronal Signal. 2017. PMID: 32714573 Free PMC article. Review. - The p75NTR neurotrophin receptor is required to organize the mature neuromuscular synapse by regulating synaptic vesicle availability.
Pérez V, Bermedo-Garcia F, Zelada D, Court FA, Pérez MÁ, Fuenzalida M, Ábrigo J, Cabello-Verrugio C, Moya-Alvarado G, Tapia JC, Valenzuela V, Hetz C, Bronfman FC, Henríquez JP. Pérez V, et al. Acta Neuropathol Commun. 2019 Sep 12;7(1):147. doi: 10.1186/s40478-019-0802-7. Acta Neuropathol Commun. 2019. PMID: 31514753 Free PMC article. - Schwann Cell Precursors; Multipotent Glial Cells in Embryonic Nerves.
Jessen KR, Mirsky R. Jessen KR, et al. Front Mol Neurosci. 2019 Mar 26;12:69. doi: 10.3389/fnmol.2019.00069. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30971890 Free PMC article. Review.
References
- Abercrombie M, Johnson ML. Quantitative histology of Wallerian degeneration I Nuclear population in rabbit sciatic nerve. J Anat. 1946;80:37–50. - PubMed
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials