Eye position signal modulates a human parietal pointing region during memory-guided movements - PubMed (original) (raw)
Eye position signal modulates a human parietal pointing region during memory-guided movements
J F DeSouza et al. J Neurosci. 2000.
Abstract
Using functional magnetic resonance imaging, we examined the signal in parietal regions that were selectively activated during delayed pointing to flashed visual targets and determined whether this signal was dependent on the fixation position of the eyes. Delayed pointing activated a bilateral parietal area in the intraparietal sulcus (rIPS), rostral/anterior to areas activated by saccades. During right-hand pointing to centrally located targets, the left rIPS region showed a significant increase in activation when the eye position was rightward compared with leftward. As expected, activation in motor cortex showed no modulation when only eye position changed. During pointing to retinotopically identical targets, the left rIPS region again showed a significant increased signal when the eye position was rightward compared with leftward. Conversely, when pointing with the left arm, the right rIPS showed an increase in signal when eye position was leftward compared with rightward. The results suggest that the human parietal hand/arm movement region (rIPS), like monkey parietal areas (Andersen et al., 1985), exhibits an eye position modulation of its activity; modulation that may be used to transform the coordinates of the retinotopically coded target position into a motor error command appropriate for the wrist.
Figures
Fig. 1.
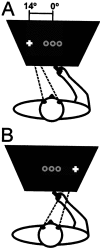
A, Schematic view of the subject laying in the magnet bore while pointing to one of three flashed target positions (represented by the gray circles at the center) and fixating the left cross or performing the same pointing movements while fixating on the_right cross_ (B).
Fig. 2.
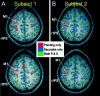
A, Two axial-oblique anatomical slices showing functional activation along the intraparietal sulcus region from a representative subject. The slices show voxels activated (p < 0.0001) during pointing only (red), during saccades only (blue), and during both pointing and saccades (green).B, A second subject. The thin light blue line outlines the central sulcus, the dotted white line outlines the postcentral sulcus, and the_yellow lines_ outline the various branches of the intraparietal sulcus in each subject. M1, Motor cortex activation. Left in each image is the left hemisphere.
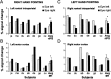
Fig. 3.
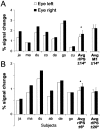
The activation produced by pointing movements of the right hand is modulated by eye position. _White bars_represent percent signal change during pointing while the eyes were fixating to the left and the black bars while fixating to the right. A, The signal activity from left rIPS when fixating 14° to the left and right in seven subjects, their average and SE (Avg rIPS ± _14_°). The far right bars plot the average (n = 7) and SE from motor cortex (Avg M1 ± _14_°). B, The signal intensity from left rIPS as the subjects change eye position from 6° to the left and the right. The average and SE for the ±6° (Avg rIPS ± _6_°) and also ±20° (Avg rIPS ± _20_°) change in eye position is plotted to the right. *p< 0.05. M1, Motor cortex activation.
Fig. 4.
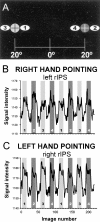
A, Target locations for eye fixation (black cross surrounded by light_or dark gray circles) and pointing positions (white ovals with numbers). The_numbers within the ovals represent the order of the pointing blocks. B, The graph plots signal intensity versus image number (1 image is 2 sec) from a representative subject's left rIPS voxels during right-hand pointing. The shading of each bar represent fixation position (light gray to the_left_, dark gray to the_right_). The numbers correspond to the pointing positions indicated in A. The white portions represent the control periods when the subject was fixating but not pointing to the flashed targets. C, Same as B but now for the right rIPS during left-hand pointing.
Fig. 5.
A, The percent signal change from voxels in the left rIPS during pointing with the right hand to retinotopically identical targets while fixating 20° to the left or right. The individual data of seven subjects as well as their average (Avg rIPS) and SE are plotted. B, The same for the left motor cortex. C, The same as_A_ except for right rIPS while pointing with the left hand (n = 6). D, The same as_C_ except for right motor cortex. *p< 0.05, **p < 0.005.
Similar articles
- A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing.
Connolly JD, Goodale MA, DeSouza JF, Menon RS, Vilis T. Connolly JD, et al. J Neurophysiol. 2000 Sep;84(3):1645-55. doi: 10.1152/jn.2000.84.3.1645. J Neurophysiol. 2000. PMID: 10980034 Clinical Trial. - Integration of target and effector information in human posterior parietal cortex for the planning of action.
Medendorp WP, Goltz HC, Crawford JD, Vilis T. Medendorp WP, et al. J Neurophysiol. 2005 Feb;93(2):954-62. doi: 10.1152/jn.00725.2004. Epub 2004 Sep 8. J Neurophysiol. 2005. PMID: 15356184 - Parietal and superior frontal visuospatial maps activated by pointing and saccades.
Hagler DJ Jr, Riecke L, Sereno MI. Hagler DJ Jr, et al. Neuroimage. 2007 May 1;35(4):1562-77. doi: 10.1016/j.neuroimage.2007.01.033. Epub 2007 Feb 8. Neuroimage. 2007. PMID: 17376706 Free PMC article. - Is reaching eye-centered, body-centered, hand-centered, or a combination?
Graziano MS. Graziano MS. Rev Neurosci. 2001;12(2):175-85. doi: 10.1515/revneuro.2001.12.2.175. Rev Neurosci. 2001. PMID: 11392457 Review. - Parietal and hippocampal contribution to topokinetic and topographic memory.
Berthoz A. Berthoz A. Philos Trans R Soc Lond B Biol Sci. 1997 Oct 29;352(1360):1437-48. doi: 10.1098/rstb.1997.0130. Philos Trans R Soc Lond B Biol Sci. 1997. PMID: 9368932 Free PMC article. Review.
Cited by
- Brain activation related to combinations of gaze position, visual input, and goal-directed hand movements.
Bédard P, Wu M, Sanes JN. Bédard P, et al. Cereb Cortex. 2011 Jun;21(6):1273-82. doi: 10.1093/cercor/bhq205. Epub 2010 Oct 25. Cereb Cortex. 2011. PMID: 20974688 Free PMC article. - Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades.
Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Schluppeck D, et al. J Neurosci. 2006 May 10;26(19):5098-108. doi: 10.1523/JNEUROSCI.5330-05.2006. J Neurosci. 2006. PMID: 16687501 Free PMC article. Clinical Trial. - Allocentric versus egocentric representation of remembered reach targets in human cortex.
Chen Y, Monaco S, Byrne P, Yan X, Henriques DY, Crawford JD. Chen Y, et al. J Neurosci. 2014 Sep 10;34(37):12515-26. doi: 10.1523/JNEUROSCI.1445-14.2014. J Neurosci. 2014. PMID: 25209289 Free PMC article. - Tracking Plasticity: Effects of Long-Term Rehearsal in Expert Dancers Encoding Music to Movement.
Bar RJ, DeSouza JF. Bar RJ, et al. PLoS One. 2016 Jan 29;11(1):e0147731. doi: 10.1371/journal.pone.0147731. eCollection 2016. PLoS One. 2016. PMID: 26824475 Free PMC article. - Eye position-dependent activity in the primary visual area as revealed by fMRI.
Andersson F, Joliot M, Perchey G, Petit L. Andersson F, et al. Hum Brain Mapp. 2007 Jul;28(7):673-80. doi: 10.1002/hbm.20296. Hum Brain Mapp. 2007. PMID: 17089375 Free PMC article.
References
- Andersen RA, Essick GK, Seigal RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230:456–458. - PubMed
- Andersen RA, Snyder LH, Li CS, Stricanne B. Coordinate transformations in the representation of spatial information. Curr Opin Neurobiol. 1993;3:171–176. - PubMed
- Andersen RA, Snyder LH, Batista AP, Buneo CA, Cohen YE. Posterior parietal areas specialized for eye movements (LIP) and reach (PRR) using a common coordinate frame. Novartis Found Symp. 1998;218:109–122. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials