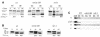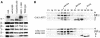COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP - PubMed (original) (raw)
COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP
A Eugster et al. EMBO J. 2000.
Abstract
We performed a systematic mapping of interaction domains on COP I subunits to gain novel insights into the architecture of coatomer. Using the two-hybrid system, we characterize the domain structure of the alpha-, beta'-, epsilon-COP and beta-, gamma-, delta-, zeta-COP coatomer subcomplexes and identify links between them that contribute to coatomer integrity. Our results demonstrate that the domain organization of the beta-, gamma-, delta-, zeta-COP subcomplex and AP adaptor complexes is related. Through in vivo analysis of alpha-COP truncation mutants, we characterize distinct functional domains on alpha-COP. Its N-terminal WD40 domain is dispensable for yeast cell viability and overall coatomer function, but is required for KKXX-dependent trafficking. The last approximately 170 amino acids of alpha-COP are also non-essential for cell viability, but required for epsilon-COP incorporation into coatomer and maintainance of normal epsilon-COP levels. Further, we demonstrate novel direct interactions of coatomer subunits with regulatory proteins: beta'- and gamma-COP interact with the ARF-GTP-activating protein (GAP) Glo3p, but not Gcs1p, and beta- and epsilon-COP interact with ARF-GTP. Glo3p also interacts with intact coatomer in vitro.
Figures
Fig. 1. A tentative domain structure of α-COP. The location of α-COP point mutations and the corresponding phenotypes of mutant α-COP strains are indicated. In this study, we show that the last ∼170 amino acids of α-COP are important for interactions with β′-COP and ε-COP, and thus refer to this region as the ‘C-terminal domain’.
Fig. 2. Novel α-COP mutants lacking the the WD40 domain or a C-terminal region are viable. GAL1::ret1Δ1–285 and GAL1::ret1Δ1034–1201 cells were streaked onto minimal plates containing either galactose (Gal) or glucose (Glu) and grown for 3 days at the temperatures indicated. Strains were, as indicated: wild-type control strain YAE7, GAL1::RET1 (YAE3), GAL1::ret1Δ1034–1201 (YAE4) and GAL1::ret1Δ1–285 (YAE5). Note that GAL1::ret1Δ1–285 cells are temperature-sensitive.
Fig. 3. (A) ret1Δ1–285 α-COP mutant cells display defects in KKXX trafficking in vivo. ret1Δ1–285 cells, congenic wild-type cells (YAE7) and ret1-1 cells were transformed with plasmids expressing Inv.-Wbp1 fusions carrying either the KKXX motif (KK) or a mutated motif, QKXX (QK). Cells were grown to log phase at 30°C, and pulse-labelled with [35S]Promix for 10 min at 30°C. Chase time points were taken at 0 and 60 min. Intact and _PEP4_-processed fusion proteins migrate at 70 and 56 kDa. The percentage of processing at 60 min was quantified using a PhosphorImager. The asterisk marks a band that may represent non-specific, cross-reactive material and was not included in the quantitation. (B) ret1Δ1–285 cells display a minor delay in CPY transport. Pulse–chase analysis of CPY transport in ret1Δ1–285 cells, congenic wild-type strain YAE7 (wt) and ret1-1 cells. Cells were radiolabelled with [35S]Promix label for 10 min at 30°C and chase time points taken at 0, 15 and 60 min. p1, p2 and mature forms of CPY are indicated, and processing to the mature form is quantified at 15 and 60 min. (C) Coatomer from ret1Δ1–285 cells is unable to bind to the KKXX motif. Whole-cell lysate of wild-type cells (WT), ret1Δ1–285 cells or ret1-1 cells was incubated for 2 h at 4°C with immobilized GST fusion proteins harbouring the KKXX motif (KK) or a mutated motif, SSXX (SS). Bound proteins were eluted from the beads, separated by SDS–PAGE and analysed by immunoblot using anti-coatomer antiserum. Purified coatomer (coat) is loaded to define positions of the COPs. Note that binding of coatomer subunits from wild-type cells, but not ret1-1 and ret1Δ1–285 cells, to the KKXX fusion protein.
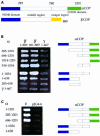
Fig. 4. (A) ε-COP levels are severely reduced in ret1Δ1034–1201 mutant cells. ret1Δ strains expressing full-length α-COP (RET1) or the α-COP truncations (ret1Δ1–285 or ret1Δ1034–1201) from a CEN plasmid under the control of the GAL1 promoter, as well as a congenic wild-type control were grown to log phase at 30°C. Total cell extracts were analysed by immunoblotting. α-COP was detetected with anti-coatomer antiserum; the other COPs were detected with subunit-specific antisera. Note the higher levels of _GAL1_-overexpressed α-COP compared with wild-type cells, as expected, and the size differences between full-length and truncated α-COP. Further, note the dramatic depletion of ε-COP in ret1Δ1034–1201 cells, and comparable levels of β-, β′-, γ- and δ-COP in all four strains. (B) In ret1Δ1034–1201 mutant cells, ε-COP is not bound to coatomer. Superose 6 gel filtration of cytosolic proteins from GAL1::RET1 cells and GAL1::ret1Δ1034–1201 cells. SDS–PAGE-separated proteins of column fractions were probed with anti-coatomer antiserum to detect α-COP, β′-, β-, γ-COP (which co-migrate as a triplet) and δ-COP; an anti-ε-COP serum was used to detect ε-COP; the asterisk marks a non-specific, cross-reactive band. To visualize the reduced amounts of ε-COP in GAL1::ret1Δ1034–1201 cells, the ε-COP lane required longer exposure than for GAL1::RET1 cells. Note that coatomer from GAL1::RET1 cells elutes with a molecular mass of 700–800 kDa (fractions 17 + 18), similar to coatomer from wild-type cells (Duden et al., 1998), and that ε-COP co-fractionates with coatomer. Additional ε-COP is present in a prominent peak (∼200 kDa), most likely containing proteolytic fragments of the overexpressed, monomeric α-COP not detectable with anti-coatomer serum (fractions 21 + 22). Note that in GAL1::ret1Δ1034–1201 cells, ε-COP is absent from the broad coatomer peak around fractions 17–20. ε-COP is present exclusively in low molecular weight fractions in monomeric form. Fraction numbers and positions of marker proteins are indicated: thyroglobulin (669 000), β-amylase (200 000) and carboanhydrase (29 000).
Fig. 5. Interactions within the α-, β′-, ε-COP subcomplex. On the horizontal axis, full-length α- and β′-COP are expressed in the bait vector; on the vertical axis, full-length α-, β′- and ε-COP are expressed in the prey vector. Empty vector is used as a control. ε-COP in the bait vector displayed high intrinsic transcriptional activation and could not be tested. Note the interactions between α-COP and β′-COP, and α-COP and ε-COP, and a novel direct interaction between β′-COP and ε-COP. Results shown are from the growth assay on minimal plates lacking leucine. As is typical of two-hybrid studies (Estojak et al., 1995), the same proteins expressed in the two different vectors (bait and prey) may show different signal intensities. Note the interaction between α-COP and β′-COP for an example.
Fig. 6. Mapping of domain interactions within the α-, β′-, ε-COP subcomplex. (A) Tentative domain structure of α- and β′-COP, based on a multiple alignment of α- and β′-COP from distant species. Both proteins harbour an N-terminal WD40 domain (∼285 residues). A middle region (residues ∼285 to ∼780 on both proteins) shares sequence similarity in α-COP and β′-COP. A ‘unique region’ (length ∼200 residues for β′-COP and ∼400 residues for α-COP) with no recognizable sequence relationship between the two proteins is present at the C-terminus. The C-terminal domain on α-COP is indicated. (B) Mapping of α-/β′-COP interactions. α-COP truncations expressed in the prey plasmid were tested against β′-COP in the bait plasmid; amino acids of α- and β′-COP encoded by the two-hybrid fusions are indicated. The N-terminal half of γ-COP served as negative control. Results shown are from the growth assay on minimal plates lacking leucine. Note that three separable regions of α-COP, comprising residues 534–1034, 1014–1201 or the N-terminal half (residues 1–638), are able to interact with β′-COP. Note further that the WD40 domain of β′-COP is not required for these interactions. (C) Mapping of α-/ε-COP interactions. α-COP truncations were expressed in the bait vector and ε-COP in the prey vector; empty prey vector was used as a control. α-COP residues encoded by the two-hybrid fusions are indicated. Results shown are from the growth assay on minimal plates lacking leucine. ε-COP interacts with the C-terminal half of α-COP. Note that the last ∼170 residues of α-COP are essential for this interaction.
Fig. 7. ret1-3 α-COP cannot interact with β′-COP and is temperature-sensitive for interaction with ε-COP. Wild-type or ret1-3 mutant α-COP C-terminal halves (residues 685–1201) were expressed in the bait vector and tested for interaction with full-length β′-COP or ε-COP in the prey vector at 30 or 37°C. Results shown are from the growth assay on minimal plates lacking leucine. Note that Ret1-3p, in contrast to wild-type α-COP, does not interact with β′-COP at either temperature. Note further that Ret1-3p interacts with ε-COP at 30°C, but not at 37°C.
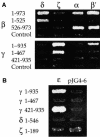
Fig. 8. Interactions of α-, β′-, ε-COP with β- and γ-COP. (A) Regions of β-COP and γ-COP involved in interactions with other coatomer subunits were mapped. The N- and C-terminal halves of β-COP and γ-COP were cloned into the prey vector pJG4-5; amino acid residues of the COPs encoded by the two-hybrid fusions are indicated. These prey fusions were tested against other coatomer subunits in the bait vector; empty pJG4-5 served as a control. Results shown are from the growth assay on minimal plates lacking leucine. Interactions with δ-, α- and β′-COP are shown in reporter strain EGY48; interactions with ζ-COP are shown using the less sensitive reporter strain EGY191. Note that the interactions of β-COP with δ-COP, and γ-COP with ζ-COP, map to the N-terminal halves of β- and γ-COP, respectively. Note further three novel interactions: β-COP with α-COP and β′-COP, and γ-COP with β′-COP. The C-terminal region of β-COP (β 526–973) interacts with α- and β′-COP; the interaction of γ-COP with β′-COP could not be mapped. (B) The N-terminal half of γ-COP interacts with ε-COP. The N- and C-terminal halves of β-COP and γ-COP were cloned into the bait vector pEG203; amino acid residues of the COPs encoded by the two-hybrid fusions are indicated. Bait fusions were tested against all other coatomer subunits in the prey vector; δ- and ζ-COP in the bait vector served as controls. Results shown are from the growth assay on minimal plates lacking leucine.
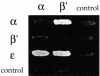
Fig. 9. (A) ARF1 interacts directly with β- and ε-COP in the two-hybrid system. N-terminally truncated (ΔN17) Arf1p in the bait vector was tested against the coatomer subunits in the prey vector. Wild-type ARF1 and the mutants Q71L (GTP-ARF), T31N (GDP-ARF) and N126I were tested. Empty prey vector was a negative control. Results shown are from the growth assay on minimal plates lacking leucine. Interactions with Q71L-ARF1, N126I-ARF1 and wild-type ARF1 are shown in reporter strain EGY48; interaction with T31N-ARF1 is shown using the less sensitive reporter strain EGY191. Note that β-COP and ε-COP interact with Q71L-ARF1 and N126I-ARF1, but not wild-type ARF1 or T31N-ARF1. (B) The ARF-GAP Glo3p interacts with β′- and γ-COP in the two-hybrid system. Glo3p or Gcs1p in the prey vector was tested against coatomer subunits in the bait vector, using empty bait vector as a control. Note that Glo3p interacts with β′- and γ-COP. (C) His6-tagged Glo3p (GLO3) can pull down whole coatomer from yeast cytosol in vitro, whereas empty beads (CTRL) or a control protein (His6-Rab-GDI; not shown), cannot. Bound proteins were analysed using anti-coatomer serum; the positions of the COPs are indicated. For details, see Materials and methods. (D) Glo3p and Gcs1p interact with Arf1p. Glo3p or Gcs1p in the prey vector was tested against wild-type or mutant ΔN17-ARF1 in the bait vector. Note that Glo3p and Gcs1p both interact with Q71L- and N126I-ARF1, but not wild-type ARF1 or T31N-ARF1. Reporter strains used were the same as in (A) above.
Fig. 10. Schematic depiction of overall topology and internal interactions within three different, but structurally related coat complexes: coatomer; AP-1 and clathrin, and AP-3 adaptor with the presumed coat proteins Vps41/Vps39p. Domain interactions of proteins within these complexes that have been identified by two-hybrid or biochemical analysis are indicated by coloured bars connecting these proteins. For details see Discussion.
Similar articles
- Multiple and stepwise interactions between coatomer and ADP-ribosylation factor-1 (Arf1)-GTP.
Sun Z, Anderl F, Fröhlich K, Zhao L, Hanke S, Brügger B, Wieland F, Béthune J. Sun Z, et al. Traffic. 2007 May;8(5):582-93. doi: 10.1111/j.1600-0854.2007.00554.x. Traffic. 2007. PMID: 17451557 - Solution structure of human zeta-COP: direct evidences for structural similarity between COP I and clathrin-adaptor coats.
Yu W, Lin J, Jin C, Xia B. Yu W, et al. J Mol Biol. 2009 Mar 6;386(4):903-12. doi: 10.1016/j.jmb.2008.12.083. Epub 2009 Jan 10. J Mol Biol. 2009. PMID: 19167404 - The alpha- and beta'-COP WD40 domains mediate cargo-selective interactions with distinct di-lysine motifs.
Eugster A, Frigerio G, Dale M, Duden R. Eugster A, et al. Mol Biol Cell. 2004 Mar;15(3):1011-23. doi: 10.1091/mbc.e03-10-0724. Epub 2003 Dec 29. Mol Biol Cell. 2004. PMID: 14699056 Free PMC article. - Peroxisome biogenesis: where Arf and coatomer might be involved.
Lay D, Gorgas K, Just WW. Lay D, et al. Biochim Biophys Acta. 2006 Dec;1763(12):1678-87. doi: 10.1016/j.bbamcr.2006.08.036. Epub 2006 Aug 30. Biochim Biophys Acta. 2006. PMID: 17023067 Review. - Arf GAPs and membrane traffic.
Nie Z, Randazzo PA. Nie Z, et al. J Cell Sci. 2006 Apr 1;119(Pt 7):1203-11. doi: 10.1242/jcs.02924. J Cell Sci. 2006. PMID: 16554436 Review.
Cited by
- Coat/Tether Interactions-Exception or Rule?
Schroeter S, Beckmann S, Schmitt HD. Schroeter S, et al. Front Cell Dev Biol. 2016 May 17;4:44. doi: 10.3389/fcell.2016.00044. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27243008 Free PMC article. Review. - A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome.
Deng Z, Chong Z, Law CS, Mukai K, Ho FO, Martinu T, Backes BJ, Eckalbar WL, Taguchi T, Shum AK. Deng Z, et al. J Exp Med. 2020 Nov 2;217(11):e20201045. doi: 10.1084/jem.20201045. J Exp Med. 2020. PMID: 32725126 Free PMC article. - Calpain 8/nCL-2 and calpain 9/nCL-4 constitute an active protease complex, G-calpain, involved in gastric mucosal defense.
Hata S, Abe M, Suzuki H, Kitamura F, Toyama-Sorimachi N, Abe K, Sakimura K, Sorimachi H. Hata S, et al. PLoS Genet. 2010 Jul 29;6(7):e1001040. doi: 10.1371/journal.pgen.1001040. PLoS Genet. 2010. PMID: 20686710 Free PMC article. - Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats.
Lee C, Goldberg J. Lee C, et al. Cell. 2010 Jul 9;142(1):123-32. doi: 10.1016/j.cell.2010.05.030. Epub 2010 Jun 24. Cell. 2010. PMID: 20579721 Free PMC article. - Early endosomes and endosomal coatomer are required for autophagy.
Razi M, Chan EY, Tooze SA. Razi M, et al. J Cell Biol. 2009 Apr 20;185(2):305-21. doi: 10.1083/jcb.200810098. Epub 2009 Apr 13. J Cell Biol. 2009. PMID: 19364919 Free PMC article.
References
- Conibear E. and Stevens,T.H. (1998) Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta, 1404, 211–230. - PubMed
- Cosson P. and Letourneur,F. (1994) Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science, 263, 1629–1631. - PubMed
- Dascher C. and Balch,W.E. (1994) Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem., 269, 1437–1448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous