Incorporation of the nuclear pore basket protein nup153 into nuclear pore structures is dependent upon lamina assembly: evidence from cell-free extracts of Xenopus eggs - PubMed (original) (raw)
Incorporation of the nuclear pore basket protein nup153 into nuclear pore structures is dependent upon lamina assembly: evidence from cell-free extracts of Xenopus eggs
C Smythe et al. EMBO J. 2000.
Abstract
In cell-free extracts of Xenopus eggs that support the assembly of replication-competent nuclei, we found that lamin B(3) specifically associates with four polypeptides (termed SLAPs, soluble lamin associated proteins). Here, one SLAP is identified as the nuclear pore complex protein Nup153, one member of the F/GXFG motif-containing nucleoporins. In vitro translated Nup153 and lamin B(3) co-immunoprecipitate, and lamin B(3) interacts specifically with the C-terminal domain of Nup153. During nuclear envelope assembly, other F/GXFG-containing nucleoporins are incorporated into the nuclear envelope preceding lamina assembly. Incorporation of Nup153 occurs at the same time as lamina assembly. When lamina assembly is prevented using the dominant-negative mutant XlaminB delta 2+, Nup153 does not appear at the nuclear envelope, while other F/GXFG-containing nucleoporins and Nup93 are recruited normally. When the lamina of pre-assembled nuclei is disrupted using the same dominant-negative mutant, the distribution of other nucleoporins is unaffected. However, Nup153 recruitment at the nuclear envelope is lost. Our results indicate that both the recruitment and maintenance of Nup153 at the pore are dependent upon the integrity of the lamina.
Figures
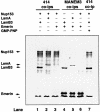
Fig. 1. Co-immunoprecipitation of Nup153 with lamin B3. Lamin B3 or PCNA was immunoprecipitated from Xenopus cytosol (USS, prepared by centrifugation of LSS at 200 000 g for 4 h) using mAb L6 5D5 or mAb PC10, respectively. Immunoprecipitates were resolved on 8% SDS–PAGE along with USS and either stained with Coomassie Blue (A) or transferred to nitrocellulose and blotted with mAb 414 (B), mAb L6 8A7 (C), mAb QE5 (D), rabbit anti-importin α (E) or rabbit anti-importin β (F). The positions of SLAPs, together with lamin B3, are indicated by arrows in (A).
Fig. 2. Co-immunoprecipitation of lamin B3 and Nup153 from rabbit reticulocyte lysates. HA-tagged Nup153, lamin B3, lamin A or emerin was transcribed and translated in rabbit reticulocyte lysates in the presence of [35S]methionine. Translation products were mixed in the absence or presence of GMP-PNP (10 mM) as indicated, immunoprecipitated with F/GXFG mAb 414 or the anti-emerin mAb MANEM3, then resolved on 8% SDS–PAGE and detected by fluorography. The relative mobilities of full-length Nup153, lamin A (LamA), lamin B3 (LamB3) and emerin are indicated with arrows. The asterisk indicates the position of the dye front.
Fig. 3. Interaction of lamin B3 with the C-terminus of Nup153 in blot overlay assays. Nup153 was expressed as three fragments (residues 2–609, 610–895 and 896–1475, respectively) in E.coli and partially purified. The fragments were resolved on SDS–PAGE and either stained with Coomassie Blue (A) or transferred to nitrocellulose and overlayed with [35S]methionine-labelled lamin B3 (B), [35S]methionine-labelled lamin A (C) or [35S]methionine-labelled emerin (D). After extensive washing, the nitrocellulose filters were dried and autoradiographed. Arrows show the positions of the full-length recombinant peptides. Molecular weight markers are 97.5, 66.3, 45, 31, 21.5 and 14.5 kDa, respectively.
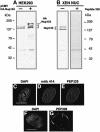
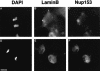
Fig. 4. Anti-PEP328 antibodies react specifically with Nup 153 and detect it at nuclear pores. Extracts of untransfected HEK293 cells (A, –), HEK293 cells transfected with HA-tagged rat Nup 153 (A, +) and isolated Xenopus sperm pro-nuclei (B) were resolved by 8% SDS–PAGE and transferred to nitrocellulose filters. The filters were blotted with either sheep anti-PEP328 (A, all lanes and B, left-hand lane) or sheep anti-PEP328 that had been pre-absorbed with PEP328 (1 mg/ml, B, right-hand lane). (C–E) HDFs were fixed after extraction with CSK-Triton and RSB-MagiK and co-stained with DAPI (C), mAb 414 (D) and anti-PEP328 (E). The distribution of sheep immunoglobulins was revealed with TRITC-conjugated rabbit anti-sheep Ig while the distribution of mouse immunoglobulins was revealed with FITC-conjugated goat anti-mouse Ig. (F and G) Xenopus sperm pronuclei were extracted with CSK-Triton and RSB-MagiK, fixed with EGS and stained with DAPI (F) and sheep anti-PEP328 followed by TRITC-conjugated rabbit anti-sheep Ig (G). In (C) and (F), the distribution of DNA is revealed with DAPI. Scale bars = 10 µm.
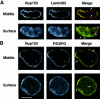
Fig. 5. Confocal microscope analysis of Nup153, lamin B3 and F/GXFG nucleoporin distribution in sperm pronuclei. Sperm pronuclei were assembled in vitro and collected on coverslips, extracted with CSK-Triton followed by RSB-MagiK and fixed. Fixed nuclei were co-stained with anti-Nup153 antibody (Nup153) and mAb L6 5D5 (LaminB3) in (A) or anti-Nup153 antibody (Nup153) and mAb 414 (F/GXFG) in (B). The distribution of mAbs was revealed with FITC-conjugated goat anti-mouse antibodies. The distribution of anti-Nup153 was revealed with TRITC-conjugated donkey anti-sheep antibodies. Nuclei were imaged on a Zeiss LCM410 equipped with a PlanApochromat ×63/1.40 N/A oil immersion lens. Optical sections were collected sequentially in each fluorescent channel at 0.4 µm intervals. Images were projected either at the middle of a nucleus or at the surface of a nucleus. Micrographs are presented either as single fluorescent (black and white) or merged images. Arrows show the positions of dot-like structures stained with anti-Nup153 antibodies. Scale bar = 20 µm.
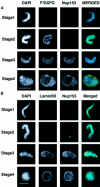
Fig. 6. Investigation of the timing of accumulation of nucleoporins, lamins and Nup 153 at the NE in assembling sperm pronuclei. Sperm pronuclei were assembled in egg extracts. (A and B) At 20 min intervals throughout the incubation, nuclei were collected on coverslips, extracted with CSK-Triton followed by RSB-MagiK and fixed. Fixed nuclei were co-stained with mAb 414 (F/GXFG) and anti-Nup153 antibody (Nup153, A) or mAb L6 5D5 (lamin B3) and anti-Nup153 antibody (Nup153, B). The distribution of mAbs was revealed with FITC-conjugated goat anti-mouse antibodies. The distribution of anti-Nup153 was revealed with TRITC-conjugated donkey anti-sheep antibodies. The distribution of chromatin was detected with DAPI. Images were collected with a CCD camera mounted on a Zeiss Axiovert 10 epifluorescence microscope. To determine the relative distributions of chromatin, F/GXFG nucleoporins, lamin B3 and Nup153, images from all three fluorescent channels were merged. Stages 1–4 illustrate morphologies typically associated with NE assembly. Scale bars = 20 µm. (C) At the times indicated, in vitro assembled nuclei were isolated as described in Materials and methods and proteins from 7.5 × 103 nuclei were subjected to electrophoresis on a 10% SDS–PAGE minigel, transferred to nitrocellulose using conditions to maximize efficiency of transfer of both high and low molecular weight proteins. Filters were blotted either with mAb 414 (left-hand panel) or anti-Nup153 antibody (right-hand panel). Under these conditions, Nup153 appears as a single band.
Fig. 6. Investigation of the timing of accumulation of nucleoporins, lamins and Nup 153 at the NE in assembling sperm pronuclei. Sperm pronuclei were assembled in egg extracts. (A and B) At 20 min intervals throughout the incubation, nuclei were collected on coverslips, extracted with CSK-Triton followed by RSB-MagiK and fixed. Fixed nuclei were co-stained with mAb 414 (F/GXFG) and anti-Nup153 antibody (Nup153, A) or mAb L6 5D5 (lamin B3) and anti-Nup153 antibody (Nup153, B). The distribution of mAbs was revealed with FITC-conjugated goat anti-mouse antibodies. The distribution of anti-Nup153 was revealed with TRITC-conjugated donkey anti-sheep antibodies. The distribution of chromatin was detected with DAPI. Images were collected with a CCD camera mounted on a Zeiss Axiovert 10 epifluorescence microscope. To determine the relative distributions of chromatin, F/GXFG nucleoporins, lamin B3 and Nup153, images from all three fluorescent channels were merged. Stages 1–4 illustrate morphologies typically associated with NE assembly. Scale bars = 20 µm. (C) At the times indicated, in vitro assembled nuclei were isolated as described in Materials and methods and proteins from 7.5 × 103 nuclei were subjected to electrophoresis on a 10% SDS–PAGE minigel, transferred to nitrocellulose using conditions to maximize efficiency of transfer of both high and low molecular weight proteins. Filters were blotted either with mAb 414 (left-hand panel) or anti-Nup153 antibody (right-hand panel). Under these conditions, Nup153 appears as a single band.
Fig. 7. The recruitment of Nup153 and lamin B3 during NE reassembly in Xenopus XLK-2 cells. Cells were fixed and co-stained with DAPI (A and D), mAb L6 5D5 (lamin B3, B and E) and anti-Nup153 antibody (Nup153, C and F) followed by secondary antibodies as described in Figure 5. Fluorescent images of cells in anaphase (A–C) and early G1 phase (D–F) were collected as described in Figure 5. Scale bar = 5 µm.
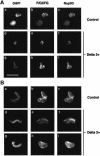
Fig. 8. The incorporation of Nup153 into the NE in nuclei treated with the dominant-negative lamin mutant Xlamin B1 Δ2+. (A) Sperm pronuclei were assembled in egg extract for 90 min in either the presence (Delta 2+) or absence (Control) of Xlamin B1 Δ2+. The nuclei were then collected on glass coverslips, extracted and fixed as described for Figure 5. The nuclei were stained with mAb 414 (F/GXFG, panels b, e, h, k, n, q) and anti-Nup153 (Nup153, panels c, f, i, l, o, r). The distribution of mAb 414 was revealed with FITC-conjugated goat anti-mouse Ig while the distribution of anti-Nup153 was revealed with TRITC-conjugated donkey anti-sheep Ig. The distribution of chromatin in each image is revealed with DAPI (panels a, d, g, j, m, p). (B) Alternatively, sperm pronuclei were assembled in egg extracts over 90 min before Xlamin B1 Δ2+ (Delta 2+) or control buffer (Control) was added to the incubation. Ninety minutes after the addition of the mutant protein, nuclei were processed for immunofluorescence as described in (A). The nuclei were stained with mAb 414 (F/GXFG, panels b, e, h, k, n, q), anti-Nup153 (Nup153, panels c, f, i, l, o, r) and DAPI (panels a, d, g, j, m, p). Scale bar = 20 µm.
Fig. 9. The incorporation of Nup93 into the NE occurs independently of the lamina. (A) Sperm pronuclei were assembled in egg extract for 90 min in either the presence (Delta 2+) or absence (Control) of Xlamin B1 Δ2+. The nuclei were then collected on glass coverslips, extracted and fixed as described for Figure 5. The nuclei were stained with mAb 414 (F/GXFG, panels b, e, h) and anti-Nup93 (Nup93, panels c, f, i,). The distribution of mAb 414 was revealed with FITC-conjugated goat anti-mouse Ig while the distribution of anti-Nup93 was revealed with TRITC-conjugated goat anti-rabbit Ig. The distribution of chromatin in each image is revealed with DAPI (panels a, d, g). (B) Alternatively, sperm pronuclei were assembled in egg extracts over 90 min before Xlamin B1 Δ2+ (Delta 2+) or control buffer (Control) was added to the incubation. Ninety minutes after the addition of the mutant protein, nuclei were processed for immunofluorescence as described in (A). The nuclei were stained with mAb 414 (F/GXFG, panels b, e, h), anti-Nup93 (Nup93, panels c, f, i) and DAPI (panels a, d, g). Scale bar = 20 µm.
Similar articles
- Investigations of the pathway of incorporation and function of lamin A in the nuclear lamina.
Dyer JA, Lane BE, Hutchison CJ. Dyer JA, et al. Microsc Res Tech. 1999 Apr 1;45(1):1-12. doi: 10.1002/(SICI)1097-0029(19990401)45:1<1::AID-JEMT1>3.0.CO;2-Z. Microsc Res Tech. 1999. PMID: 10206150 - The nucleoporin Nup153 maintains nuclear envelope architecture and is required for cell migration in tumor cells.
Zhou L, Panté N. Zhou L, et al. FEBS Lett. 2010 Jul 16;584(14):3013-20. doi: 10.1016/j.febslet.2010.05.038. Epub 2010 May 24. FEBS Lett. 2010. PMID: 20561986 - Evidence for the direct involvement of lamins in the assembly of a replication competent nucleus.
Jenkins H, Whitfield WG, Goldberg MW, Allen TD, Hutchison CJ. Jenkins H, et al. Acta Biochim Pol. 1995;42(2):133-43. Acta Biochim Pol. 1995. PMID: 8588455 - Function and assembly of nuclear pore complex proteins.
Bodoor K, Shaikh S, Enarson P, Chowdhury S, Salina D, Raharjo WH, Burke B. Bodoor K, et al. Biochem Cell Biol. 1999;77(4):321-9. Biochem Cell Biol. 1999. PMID: 10546895 Review. - Nuclear lamins: their structure, assembly, and interactions.
Stuurman N, Heins S, Aebi U. Stuurman N, et al. J Struct Biol. 1998;122(1-2):42-66. doi: 10.1006/jsbi.1998.3987. J Struct Biol. 1998. PMID: 9724605 Review.
Cited by
- Xenopus LAP2β protein knockdown affects location of lamin B and nucleoporins and has effect on assembly of cell nucleus and cell viability.
Dubińska-Magiera M, Chmielewska M, Kozioł K, Machowska M, Hutchison CJ, Goldberg MW, Rzepecki R. Dubińska-Magiera M, et al. Protoplasma. 2016 May;253(3):943-956. doi: 10.1007/s00709-015-0861-y. Epub 2015 Jul 25. Protoplasma. 2016. PMID: 26209045 Free PMC article. - Cytoplasmic nucleoporin assemblage: the cellular artwork in physiology and disease.
Lin J, Sumara I. Lin J, et al. Nucleus. 2024 Dec;15(1):2387534. doi: 10.1080/19491034.2024.2387534. Epub 2024 Aug 12. Nucleus. 2024. PMID: 39135336 Free PMC article. Review. - The nuclear pore complex.
Adam SA. Adam SA. Genome Biol. 2001;2(9):REVIEWS0007. doi: 10.1186/gb-2001-2-9-reviews0007. Epub 2001 Aug 31. Genome Biol. 2001. PMID: 11574060 Free PMC article. Review. - Emerin Is Required for Proper Nucleus Reassembly after Mitosis: Implications for New Pathogenetic Mechanisms for Laminopathies Detected in EDMD1 Patients.
Dubińska-Magiera M, Kozioł K, Machowska M, Piekarowicz K, Filipczak D, Rzepecki R. Dubińska-Magiera M, et al. Cells. 2019 Mar 13;8(3):240. doi: 10.3390/cells8030240. Cells. 2019. PMID: 30871242 Free PMC article. - A statistical image analysis framework for pore-free islands derived from heterogeneity distribution of nuclear pore complexes.
Mimura Y, Takemoto S, Tachibana T, Ogawa Y, Nishimura M, Yokota H, Imamoto N. Mimura Y, et al. Sci Rep. 2017 Nov 24;7(1):16315. doi: 10.1038/s41598-017-16386-2. Sci Rep. 2017. PMID: 29176624 Free PMC article.
References
- Blow J.J. and Laskey,R.A. (1986) Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell, 47, 577–587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources