Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens - PubMed (original) (raw)
Review
Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens
C Nathan et al. Proc Natl Acad Sci U S A. 2000.
Abstract
This review summarizes recent evidence from knock-out mice on the role of reactive oxygen intermediates and reactive nitrogen intermediates (RNI) in mammalian immunity. Reflections on redundancy in immunity help explain an apparent paradox: the phagocyte oxidase and inducible nitric oxide synthase are each nonredundant, and yet also mutually redundant, in host defense. In combination, the contribution of these two enzymes appears to be greater than previously appreciated. The remainder of this review focuses on a relatively new field, the basis of microbial resistance to RNI. Experimental tuberculosis provides an important example of an extended, dynamic balance between host and pathogen in which RNI play a major role. In diseases such as tuberculosis, a molecular understanding of host-pathogen interactions requires characterization of the defenses used by microbes against RNI, analogous to our understanding of defenses against reactive oxygen intermediates. Genetic and biochemical approaches have identified candidates for RNI-resistance genes in Mycobacterium tuberculosis and other pathogens.
Figures
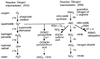
Figure 1
ROI and RNI production in mammalian cells via phox and NOS: parallel but connecting paths. Nitroxyl anion (NO−), a one-electron reduction product of nitric oxide (⋅NO), is unlikely to arise from ⋅NO under physiologic conditions, but is considered by some investigators to be a primary and more toxic product of NOS (91). Reaction of RNI with cysteine sulfhydryls can lead either to_S_-nitrosylation or to oxidation to the sulfenic acid, as well as to disulfide bond formation (not shown), all of which are potentially reversible. Peroxynitrite anion (OONO−) and peroxynitrous acid (OONOH) have distinct patterns of reactivity (92), but for simplicity, the text refers to both as peroxynitrite. OONOH spontaneously decomposes via species resembling the reactive radicals, hydroxyl (OH⋅) and/or nitrogen dioxide (⋅NO2). When
l
-arginine is limiting, NOS can produce superoxide (O2⨪) along with ⋅NO, favoring the formation of peroxynitrite (5).
Similar articles
- The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis.
Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR Jr, Chan J. Ohno H, et al. Cell Microbiol. 2003 Sep;5(9):637-48. doi: 10.1046/j.1462-5822.2003.00307.x. Cell Microbiol. 2003. PMID: 12925133 - The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice.
Scanga CA, Mohan VP, Tanaka K, Alland D, Flynn JL, Chan J. Scanga CA, et al. Infect Immun. 2001 Dec;69(12):7711-7. doi: 10.1128/IAI.69.12.7711-7717.2001. Infect Immun. 2001. PMID: 11705952 Free PMC article. - Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria.
Shiloh MU, Nathan CF. Shiloh MU, et al. Curr Opin Microbiol. 2000 Feb;3(1):35-42. doi: 10.1016/s1369-5274(99)00048-x. Curr Opin Microbiol. 2000. PMID: 10679417 Review. - Reactive nitrogen and oxygen intermediates and bacterial defenses: unusual adaptations in Mycobacterium tuberculosis.
Zahrt TC, Deretic V. Zahrt TC, et al. Antioxid Redox Signal. 2002 Feb;4(1):141-59. doi: 10.1089/152308602753625924. Antioxid Redox Signal. 2002. PMID: 11970850 Review.
Cited by
- Cell-autonomous targeting of arabinogalactan by host immune factors inhibits mycobacterial growth.
Qin L, Xu J, Chen J, Wang S, Zheng R, Cui Z, Liu Z, Wu X, Wang J, Huang X, Wang Z, Wang M, Pan R, Kaufmann SHE, Meng X, Zhang L, Sha W, Liu H. Qin L, et al. Elife. 2024 Nov 4;13:RP92737. doi: 10.7554/eLife.92737. Elife. 2024. PMID: 39495223 Free PMC article. - Metabolic host responses to infection by intracellular bacterial pathogens.
Eisenreich W, Heesemann J, Rudel T, Goebel W. Eisenreich W, et al. Front Cell Infect Microbiol. 2013 Jul 9;3:24. doi: 10.3389/fcimb.2013.00024. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23847769 Free PMC article. Review. - Hybrid cluster proteins and flavodiiron proteins afford protection to Desulfovibrio vulgaris upon macrophage infection.
Figueiredo MC, Lobo SA, Sousa SH, Pereira FP, Wall JD, Nobre LS, Saraiva LM. Figueiredo MC, et al. J Bacteriol. 2013 Jun;195(11):2684-90. doi: 10.1128/JB.00074-13. Epub 2013 Apr 5. J Bacteriol. 2013. PMID: 23564166 Free PMC article. - Resistance to first-line anti-TB drugs is associated with reduced nitric oxide susceptibility in Mycobacterium tuberculosis.
Idh J, Mekonnen M, Abate E, Wedajo W, Werngren J, Ängeby K, Lerm M, Elias D, Sundqvist T, Aseffa A, Stendahl O, Schön T. Idh J, et al. PLoS One. 2012;7(6):e39891. doi: 10.1371/journal.pone.0039891. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768155 Free PMC article. - Reactive oxygen species and nitric oxide in cutaneous leishmaniasis.
Horta MF, Mendes BP, Roma EH, Noronha FS, Macêdo JP, Oliveira LS, Duarte MM, Vieira LQ. Horta MF, et al. J Parasitol Res. 2012;2012:203818. doi: 10.1155/2012/203818. Epub 2012 Apr 12. J Parasitol Res. 2012. PMID: 22570765 Free PMC article.
References
- Klebanoff S J. In: Inflammation. Gallin J I, Snyderman R, Fearon D T, Haynes B F, Nathan C, editors. Philadelphia: Lippincott; 1999. pp. 721–768.
- Nathan C. FASEB J. 1992;6:3051–3064. - PubMed
- Ischiropoulos H, Zhu L, Beckman J S. Arch Biochem Biophys. 1992;298:446–451. - PubMed
- Zingarelli B, O'Connor M, Wong H, Salzman A L, Szabo C. J Immunol. 1996;156:350–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources