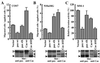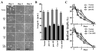Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation - PubMed (original) (raw)
Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation
H Yano et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Protein tyrosine phosphorylation accompanies and is essential for integrin signaling. We have shown that tyrosine phosphorylation of paxillin alpha and Crk-associated substrate (p130(Cas)) is a prominent event on integrin activation in normal murine mammary gland epithelial cells. Tyrosine phosphorylation of p130(Cas) has been demonstrated to facilitate cell migration. We show here that tyrosine phosphorylation of paxillin alpha acts to reduce haptotactic cell migrations as well as transcellular invasive activities in several different experimental cell systems, whereas tyrosine phosphorylation of p130(Cas) exerts opposing effects to those of paxillin alpha. Each of the phosphorylation-null mutants acts as a dominant negative for each phenotype. Moreover, we found that overexpression of paxillin alpha reduced the cell saturation density of normal murine mammary gland cells, whereas overexpression of p130(Cas) increased it. These effects also seemed to depend on tyrosine phosphorylation events. Cell growth rates and morphologies at growing phases were not significantly altered, nor were cells transformed. Addition of epidermal growth factor increased saturation density of the paxillin alpha-overexpressing cells, whereas no further increment was observed in p130(Cas)-overexpressing cells. We propose that tyrosine phosphorylation of paxillin alpha and p130(Cas) exerts opposing effects on several integrin-mediated cellular events, possibly through different signaling pathways.
Figures
Figure 1
Opposing effects of paxillin α and p130Cas on cell migratory activities through tyrosine phosphorylation. (A and B) Haptotactic transmigrating activities toward collagen type I by using modified Boyden chambers. Serum-starved COS7 cells (A) or serum-starved NMuMG cells (B) were allowed to migrate for 3 h on collagen-coated membrane after transfection with either the empty expression vector (vector) or the expression vector containing cDNA for EGFP-paxillin α wt (pax wt), its 4X mutant (pax 4X), EGFP-p130Cas wt (Cas wt), or its ΔSD mutant (Cas ΔSD). COS7 cells were transiently transfected by using FuGENE6 with an efficiency of 40–60%. NMuMG cells were stably transfected by retrovirus infection. Each bar represents the mean +/− SEM of triplicate of three independent experiments. (C) Transcellular invasion activities through mesothelial cell monolayer. LPA-stimulated MM1 cells cultured in the presence of 10% serum, stably expressing each cDNA as indicated, were seeded onto the mesothelial cell monolayer and allowed to migrate for 20 h without serum. Invasive activities of each transfectant were shown as relative activities to the vector control cells. Each bar represents the mean +/− SEM of triplicate of an independent experiments. Levels of exogenous proteins (open arrowheads) compared with endogenous proteins (closed arrowheads), as assessed by immunoblotting by using antipaxillin and anti-p130Cas, respectively, were also shown below (A–C).
Figure 2
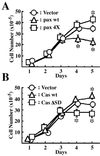
Effects of EGFP-paxillin α, EGFP-p130Cas, and their tyrosine phosphorylation null mutants on cell saturation densities. Each 2 × 105 cells were plated on 6-cm dishes and cultured for 5 days in the presence of serum. Cell numbers were measured every day by collecting cells and counting the viable cells. The same sets of NMuMG cells were used as in Fig. 1. Duplicate dishes were counted for each time point of the growth curves, and each determination represents the average of three independent experiments. Error bars represent the SEM. *, P < 0.05, as compared with the vector control cells.
Figure 3
Properties of NMuMG cells expressing EGFP-paxillin α, EGFP-p130Cas, and their tyrosine phosphorylation null mutants. The same transfectants of NMuMG cells were used as in Fig. 2. (A) Phase-contrast microscopic images of same cells at days 1, 3, and 5, as in Fig. 3. Note that initial confluence was observed on day 3 with all of the transfectants. Piling up of cells was never observed even in prolonged culture more than 5 days. Bars = 100 μm. (B) Cell sizes measured at sparse cultures (day 1 as shown in Fig. 3). Mean lengths of long and short axis of cells are shown by hatched and solid bars, respectively. (C) BrdUrd incorporation of cells. Cells were incubated with 10 μM BrdUrd for 3 h at time, indicated, and then fixed. BrdUrd-positive cells were then visualized by using anti-BrdUrd antibody. Ratio of the BrdUrd-positive cells was calculated by visualizing nuclear DNA of all cell populations by using 4′,6-diamidino-2′-phenylindole dihydrochloride. Each determination represents the average of three independent experiments, and error bars represent the SEM.
Figure 4
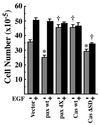
EGF increases cell saturation density of NMuMG cells expressing paxillin α. The same sets of NMuMG cells were used as in Fig. 2. Each 2 × 105 cells were plated on 6-cm dishes and cultured for 5 days in the presence of serum with (solid bar) or without (hatched bar) 10 nM EGF, and then viable cell numbers were counted. *, P < 0.05, †, P < 0.01 against values of the vector control.
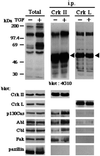
Figure 5
Paxillin does not stably associate with c-Crk proteins in NMuMG cells. NMuMG cells were treated or untreated with 2 ng/ml TGF-β for 3 days and then solubilized in 1% Nonidet P-40 buffer (27). Anti-Crk-I/Crk-II or anti-Crk-L immunoprecipitants were prepared from each 500 μg of 1% Nonidet P-40 cell lysates, separated by SDS/PAGE (8% gel), and subjected to sequential immunoblotting analysis by using antibodies as indicated. NMuMG cells expressed both Crk-I and Crk-II proteins (not shown). Total cell lysates (20 μg each) were also included (Total). Arrowheads indicate crossreaction of the secondary antibody with the heavy chain of anti-Crk antibodies used for immunoprecipitation.
Similar articles
- Activation of m3 muscarinic receptors induces rapid tyrosine phosphorylation of p125(FAK), p130(cas), and paxillin in rat pancreatic acini.
Rosado JA, Salido GM, García LJ. Rosado JA, et al. Arch Biochem Biophys. 2000 May 1;377(1):85-94. doi: 10.1006/abbi.2000.1761. Arch Biochem Biophys. 2000. PMID: 10775445 - Tyrosine phosphorylation of p125(Fak), p130(Cas), and paxillin does not require extracellular signal-regulated kinase activation in Swiss 3T3 cells stimulated by bombesin or platelet-derived growth factor.
Leopoldt D, Yee HF Jr, Saab S, Rozengurt E. Leopoldt D, et al. J Cell Physiol. 2000 May;183(2):208-20. doi: 10.1002/(SICI)1097-4652(200005)183:2<208::AID-JCP7>3.0.CO;2-5. J Cell Physiol. 2000. PMID: 10737896 - Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration.
Panetti TS. Panetti TS. Front Biosci. 2002 Jan 1;7:d143-50. doi: 10.2741/A771. Front Biosci. 2002. PMID: 11779709 Review. - Tyrosine phosphorylation in the action of neuropeptides and growth factors.
Rozengurt E, Rodríguez-Fernández JL. Rozengurt E, et al. Essays Biochem. 1997;32:73-86. Essays Biochem. 1997. PMID: 9493012 Review.
Cited by
- Adaptor protein Crk is required for ephrin-B1-induced membrane ruffling and focal complex assembly of human aortic endothelial cells.
Nagashima K, Endo A, Ogita H, Kawana A, Yamagishi A, Kitabatake A, Matsuda M, Mochizuki N. Nagashima K, et al. Mol Biol Cell. 2002 Dec;13(12):4231-42. doi: 10.1091/mbc.e02-04-0181. Mol Biol Cell. 2002. PMID: 12475948 Free PMC article. - Urinary-type plasminogen activator receptor (uPAR) modulates oral cancer cell behavior with alteration in p130cas.
Shi Z, Liu Y, Johnson JJ, Stack MS. Shi Z, et al. Mol Cell Biochem. 2011 Nov;357(1-2):151-61. doi: 10.1007/s11010-011-0885-3. Epub 2011 Jun 1. Mol Cell Biochem. 2011. PMID: 21630091 - Essential role for paxillin tyrosine phosphorylation in LPS-induced mitochondrial fission, ROS generation and lung endothelial barrier loss.
Fu P, Epshtein Y, Ramchandran R, Mascarenhas JB, Cress AE, Jacobson J, Garcia JGN, Natarajan V. Fu P, et al. Sci Rep. 2021 Sep 2;11(1):17546. doi: 10.1038/s41598-021-97006-y. Sci Rep. 2021. PMID: 34475475 Free PMC article. - Paxillin phosphorylation and complexing with Erk and FAK are regulated by PLD activity in MDA-MB-231 cells.
Pribic J, Brazill D. Pribic J, et al. Cell Signal. 2012 Aug;24(8):1531-40. doi: 10.1016/j.cellsig.2012.03.015. Epub 2012 Mar 28. Cell Signal. 2012. PMID: 22481092 Free PMC article. - Focal adhesion kinase-related proline-rich tyrosine kinase 2 and focal adhesion kinase are co-overexpressed in early-stage and invasive ErbB-2-positive breast cancer and cooperate for breast cancer cell tumorigenesis and invasiveness.
Behmoaram E, Bijian K, Jie S, Xu Y, Darnel A, Bismar TA, Alaoui-Jamali MA. Behmoaram E, et al. Am J Pathol. 2008 Nov;173(5):1540-50. doi: 10.2353/ajpath.2008.080292. Epub 2008 Oct 2. Am J Pathol. 2008. PMID: 18832579 Free PMC article.
References
- Hynes R O. Cell. 1992;69:11–25. - PubMed
- Lauffenburger D A, Horwitz A, F. Cell. 1996;84:359–369. - PubMed
- Sheetz M P, Felsenfeld D P, Galbraith C G. Trends Cell Biol. 1998;8:51–54. - PubMed
- Nakamura, K., Yano, H., Uchida, H., Hashimoto, S., Schaefer, E. & Sabe, H. J. (2000) J. Biol. Chem., in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources