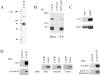Characterization of palladin, a novel protein localized to stress fibers and cell adhesions - PubMed (original) (raw)
Characterization of palladin, a novel protein localized to stress fibers and cell adhesions
M M Parast et al. J Cell Biol. 2000.
Abstract
Here, we describe the identification of a novel phosphoprotein named palladin, which colocalizes with alpha-actinin in the stress fibers, focal adhesions, cell-cell junctions, and embryonic Z-lines. Palladin is expressed as a 90-92-kD doublet in fibroblasts and coimmunoprecipitates in a complex with alpha-actinin in fibroblast lysates. A cDNA encoding palladin was isolated by screening a mouse embryo library with mAbs. Palladin has a proline-rich region in the NH(2)-terminal half of the molecule and three tandem Ig C2 domains in the COOH-terminal half. In Northern and Western blots of chick and mouse tissues, multiple isoforms of palladin were detected. Palladin expression is ubiquitous in embryonic tissues, and is downregulated in certain adult tissues in the mouse. To probe the function of palladin in cultured cells, the Rcho-1 trophoblast model was used. Palladin expression was observed to increase in Rcho-1 cells when they began to assemble stress fibers. Antisense constructs were used to attenuate expression of palladin in Rcho-1 cells and fibroblasts, and disruption of the cytoskeleton was observed in both cell types. At longer times after antisense treatment, fibroblasts became fully rounded. These results suggest that palladin is required for the normal organization of the actin cytoskeleton and focal adhesions.
Figures
Figure 1
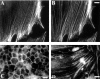
Immunofluorescent labeling with the C10 mAb. Cells were immunolabeled with C10 (A, C, and D) or fluorescent phalloidin (B). A and B, Cultured CEFs. C, Chick pigmented epithelium, in an en face preparation on gelatin-coated coverslips. D, Embryonic cardiac myocytes isolated from a day-10 chick heart. Bars: (B) 8 μm; (C) 20 μm; (D) 25 μm.
Figure 3
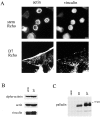
The C10 mAb immunoprecipitates a cytoskeleton-associated protein in lysates of fibroblasts. A, Immunoprecipitation from 35S-labeled CEFs. Metabolically labeled fibroblasts were trypsinized and lysed on ice, and were then centrifuged. The supernatant was subjected to IP with the C10 mAb. The major band resolves as a broad doublet at 90–92 kD. B, The C10 antibody does not cross-react with α-actinin. C10 immunoprecipitates, purified α-actinin, and whole-cell lysate from cultured CEFs were resolved by SDS-PAGE and blotted with the anti-C10 mAb, 7C6. Note that 7C6 failed to detect purified α-actinin in the middle lane. C, Analysis of the fractionation of the C10 antigen. MEF lysates were extracted with 0.5% Triton X-100 in cytoskeleton-stabilizing buffer (see Materials and Methods) and the insoluble pellet was collected by centrifugation at 14,000 rpm. 20 μg of protein from pellets and supernatants was loaded and blotted with mAb 7C6. D, C10 antigen coimmunoprecipitates with α-actinin. 3T3 cells were scraped in lysis buffer on ice, the supernatant was precleared with Gamma-bind beads alone, and mAb 1E6 was conjugated to Gamma-bind beads, and was then used to immunoprecipitate the C10 antigen. Both sets of beads were eluted and blotted with either mAb 7C6 or anti–α-actinin mAb (left). To demonstrate the specificity of the coimunoprecipitation, the IPs were also blotted for talin, vinculin, and zyxin (all negative; center). In the reverse experiment, α-actinin was immunoprecipitated using a rabbit polyclonal antibody, and the IP was blotted for α-actinin and for the C10 antigen (right).
Figure 2
Colocalization of the C10 antigen and α-actinin in stress fibers. Cultured fibroblasts were stained with C10 mAb (A and D, green fluorescence) or polyclonal anti–α-actinin (C and F, red fluorescence). The merged images (B and E) show that the C10 antigen largely colocalizes with α-actinin in a punctate pattern along stress fibers and in a concentration at the ends of stress fibers. Bars, 5 μm.
Figure 4
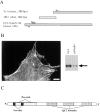
Molecular cloning and characterization of the C10 antigen. A, The pooled mAbs recognized a clone (7a-1) from an 11.5-d mouse embryo library and also a clone (18b-1) from a 10-d chick embryo library. A thorough BLAST search of the EST database identified a 3-kb EST from mouse embryo heart. The indicated primers (based on 7a-1 and the mouse EST) were used to perform RT-PCR on mouse mRNA. The start methionine with the Kozak sequence is shown. B, The full-length clone localizes properly and is recognized by anti-C10 antibodies. The myc-tagged construct was transfected into Swiss 3T3 cells, which were fixed and stained with an antimyc antibody. Note that the construct localized in focal adhesions, at the ends of stress fibers, and in a punctate pattern along the stress fibers. The transfected cells were also lysed and analyzed by Western blot. The same band is recognized by both antimyc and anti-C10 mAb 7C6. Bar, 10 μm. C, Cartoon of palladin protein. Domains identified using various programs, including proline-rich and serine-rich regions, are indicated. D, Protein sequence of palladin. Serine-rich areas are indicated with asterisks (*). Proline-rich motifs homologous to zyxin and VASP/Mena family members are underlined. The three tryptic peptides are indicated in bold-type, and the three IgC2 domains are indicated with carets (^). The stop codon is indicated by the ampersand (&).
Figure 4
Molecular cloning and characterization of the C10 antigen. A, The pooled mAbs recognized a clone (7a-1) from an 11.5-d mouse embryo library and also a clone (18b-1) from a 10-d chick embryo library. A thorough BLAST search of the EST database identified a 3-kb EST from mouse embryo heart. The indicated primers (based on 7a-1 and the mouse EST) were used to perform RT-PCR on mouse mRNA. The start methionine with the Kozak sequence is shown. B, The full-length clone localizes properly and is recognized by anti-C10 antibodies. The myc-tagged construct was transfected into Swiss 3T3 cells, which were fixed and stained with an antimyc antibody. Note that the construct localized in focal adhesions, at the ends of stress fibers, and in a punctate pattern along the stress fibers. The transfected cells were also lysed and analyzed by Western blot. The same band is recognized by both antimyc and anti-C10 mAb 7C6. Bar, 10 μm. C, Cartoon of palladin protein. Domains identified using various programs, including proline-rich and serine-rich regions, are indicated. D, Protein sequence of palladin. Serine-rich areas are indicated with asterisks (*). Proline-rich motifs homologous to zyxin and VASP/Mena family members are underlined. The three tryptic peptides are indicated in bold-type, and the three IgC2 domains are indicated with carets (^). The stop codon is indicated by the ampersand (&).
Figure 5
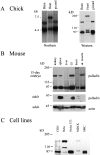
A, Palladin exists as multiple size variants. Left, Northern blot of poly-A+ RNA isolated from brain, heart, or gizzard of a day-10 chick embryo and probed under high-stringency conditions using the chick clone 18b-1 as probe. Right, Western blot of protein extracted from the same tissues, blotted using anti-C10 mAb 1E6. For every size of palladin mRNA detected in the Northern blot, a corresponding size of protein was identified. B, Palladin expression is developmentally regulated in certain tissues. Embryonic and adult mouse tissues were extracted as described in Materials and Methods. 30 μg of protein was loaded in each lane and blotted with antipalladin mAb 7C6 or an mAb to actin. Note that palladin expression is ubiquitous in the embryo and restricted to a small number of tissues in the adult. C, Palladin isoforms are detected in cultured cells. Whole cell lysates were prepared as described in Materials and Methods. 30 μg of protein was loaded in each lane and blotted with antipalladin mAb 7C6.
Figure 6
Rcho-1 cells upregulate the expression of palladin, concomitant with the formation of stress fibers and focal adhesions. A, Proliferative Rcho-1 stem cells have no organized actin stress fibers; when induced to differentiate, the cells assemble numerous stress fibers and large focal adhesions. Bar, 10 μm. B, Expression of actin, α-actinin, and vinculin does not change when Rcho-1 cells differentiate. Whole cell lysates were prepared by scraping cells in boiling Laemmli sample buffer; equal amounts of lysates were loaded in each lane and blotted for the respective protein. C, Palladin expression increases in differentiating cells. Whole cell lysates were prepared as in B and equal amounts loaded in each lane and blotted with antipalladin mAb 7C6. Only the 90–92-kD isoform was detected. D3 and D7 refer to 3 and 7 d after differentiation.
Figure 7
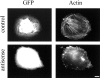
Rcho-1 cells fail to make stress fibers when treated with palladin antisense. A GFP vector was cotransfected either with an empty pAdlox vector (top) or with pAdlox–palladin antisense construct (bottom) into Rcho-1 stem cells. 2-d later, the cells were induced to differentiate. Three days after differentiation, the cells were fixed and stained with Texas red-conjugated phalloidin. Representative transfected cells are shown. Note that in the presence of the antisense construct, the cells have no stress fibers. Bar, 10 μm.
Figure 8
Reduced expression of palladin in MEFs results in a loss of stress fibers and focal adhesions. A, Adenovirus was used to deliver palladin antisense. Increasing concentrations of antisense virus leads to decreased palladin expression in MEFs. 3 d after infection, cell lysates were prepared by scraping cells in boiling Laemmli sample buffer. Blots were stained with antipalladin mAb 7C6 and antiactin (to check for equal loading). B, Increasing concentrations of antisense virus results in a loss of stress fibers and in cell rounding. MEFs were infected either with control adenovirus (empty virus or GFP-expressing virus) or with palladin antisense virus. 3 d after infection, cells were fixed and stained with Texas red-conjugated phalloidin. Bar, 20 μm.
Figure 9
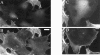
Loss of palladin expression results in a loss of stress fibers. At 2 d after infection, at the lowest virus concentration (2 × 107 pfu/ml), cells were fixed and stained with a 1:1 mixture of antipalladin mAbs 7C6 and 1E6 (A and C), and phalloidin (B and D). In each field examined, the cells with the lowest level of palladin staining exhibited a more round morphology and a lack of robust stress fibers. Bars: (A and C) 10 μm; (B and D) 5 μm.
Figure 10
Cells with attenuated palladin expression have only peripheral focal adhesions. At 2 d after infection (as in Fig. 9), cells were fixed and stained for palladin with a 1:1 mixture of mAbs 7C6 and 1E6 (A and C) and double-labeled for paxillin (B and D), as a focal adhesion marker. Cells with the lowest level of palladin staining consistently exhibited a clearing of focal adhesions from the center of the cell, such that only peripheral focal adhesions remained. Bars: (A and C) 10 μm; (B and D) 5 μm.
Similar articles
- Palladin regulates cell and extracellular matrix interaction through maintaining normal actin cytoskeleton architecture and stabilizing beta1-integrin.
Liu XS, Luo HJ, Yang H, Wang L, Kong H, Jin YE, Wang F, Gu MM, Chen Z, Lu ZY, Wang ZG. Liu XS, et al. J Cell Biochem. 2007 Apr 1;100(5):1288-300. doi: 10.1002/jcb.21126. J Cell Biochem. 2007. PMID: 17115415 - Palladin is a novel binding partner for Ena/VASP family members.
Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA. Boukhelifa M, et al. Cell Motil Cytoskeleton. 2004 May;58(1):17-29. doi: 10.1002/cm.10173. Cell Motil Cytoskeleton. 2004. PMID: 14983521 - Characterization of human palladin, a microfilament-associated protein.
Mykkänen OM, Grönholm M, Rönty M, Lalowski M, Salmikangas P, Suila H, Carpén O. Mykkänen OM, et al. Mol Biol Cell. 2001 Oct;12(10):3060-73. doi: 10.1091/mbc.12.10.3060. Mol Biol Cell. 2001. PMID: 11598191 Free PMC article. - The role of palladin in actin organization and cell motility.
Goicoechea SM, Arneman D, Otey CA. Goicoechea SM, et al. Eur J Cell Biol. 2008 Sep;87(8-9):517-25. doi: 10.1016/j.ejcb.2008.01.010. Epub 2008 Mar 14. Eur J Cell Biol. 2008. PMID: 18342394 Free PMC article. Review. - The palladin/myotilin/myopalladin family of actin-associated scaffolds.
Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. Otey CA, et al. Int Rev Cytol. 2005;246:31-58. doi: 10.1016/S0074-7696(05)46002-7. Int Rev Cytol. 2005. PMID: 16164966 Review.
Cited by
- A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: a common link for Z-disc myopathies.
von Nandelstadh P, Ismail M, Gardin C, Suila H, Zara I, Belgrano A, Valle G, Carpen O, Faulkner G. von Nandelstadh P, et al. Mol Cell Biol. 2009 Feb;29(3):822-34. doi: 10.1128/MCB.01454-08. Epub 2008 Dec 1. Mol Cell Biol. 2009. PMID: 19047374 Free PMC article. - Cytoplasmic Ig-domain proteins: cytoskeletal regulators with a role in human disease.
Otey CA, Dixon R, Stack C, Goicoechea SM. Otey CA, et al. Cell Motil Cytoskeleton. 2009 Aug;66(8):618-34. doi: 10.1002/cm.20385. Cell Motil Cytoskeleton. 2009. PMID: 19466753 Free PMC article. Review. - Palladin is upregulated in kidney disease and contributes to epithelial cell migration after injury.
Chang EH, Gasim AH, Kerber ML, Patel JB, Glaubiger SA, Falk RJ, Jennette JC, Otey CA. Chang EH, et al. Sci Rep. 2015 Jan 9;5:7695. doi: 10.1038/srep07695. Sci Rep. 2015. PMID: 25573828 Free PMC article. - Expression, crystallization and preliminary X-ray studies of the immunoglobulin-like domain 3 of human palladin.
Liang W, Yang H, Xue X, Huang Q, Bartlam M, Chen S. Liang W, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006 Jun 1;62(Pt 6):556-8. doi: 10.1107/S1744309106016411. Epub 2006 May 31. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006. PMID: 16754980 Free PMC article. - Suppression of TGFβ-mediated conversion of endothelial cells and fibroblasts into cancer associated (myo)fibroblasts via HDAC inhibition.
Kim DJ, Dunleavey JM, Xiao L, Ollila DW, Troester MA, Otey CA, Li W, Barker TH, Dudley AC. Kim DJ, et al. Br J Cancer. 2018 May;118(10):1359-1368. doi: 10.1038/s41416-018-0072-3. Epub 2018 Apr 26. Br J Cancer. 2018. PMID: 29695769 Free PMC article.
References
- Aberle H., Schwartz H., Kemler R. Cadherin–catenin complexprotein interactions and their implication for cadherin function. J. Cell. Biochem. 1996;61:514–523. - PubMed
- Arber S., Halder G., Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79:221–231. - PubMed
- Arber S., Hunter J.J., Ross J., Hongo M., Sansig G., Borg J., Perriard J.-C., Chien K.R., Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy and heart failure. Cell. 1997;88:393–403. - PubMed
- Beckerle M. Spatial control of actin filament assemblylessons from Listeria . Cell. 1998;95:741–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD034807/HD/NICHD NIH HHS/United States
- R01 GM029860/GM/NIGMS NIH HHS/United States
- GM50974/GM/NIGMS NIH HHS/United States
- HD34807/HD/NICHD NIH HHS/United States
- GM29860/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous