Protein kinase-A dependent phosphorylation of transcription enhancer factor-1 represses its DNA-binding activity but enhances its gene activation ability - PubMed (original) (raw)
Protein kinase-A dependent phosphorylation of transcription enhancer factor-1 represses its DNA-binding activity but enhances its gene activation ability
M P Gupta et al. Nucleic Acids Res. 2000.
Abstract
The cAMP-dependent signaling pathway has been implicated in cardiac cell growth/differentiation and muscle gene transcription. Previously, we have identified a cAMP-inducible E-box/M-CAT hybrid motif in the cardiac alpha-myosin heavy chain (alpha-MHC) gene promoter. The two factors, TEF-1 and Max, that bind to this motif are found to physically associate with each other and exert a positive cooperative effect for gene regulation. Here we show that TEF-1, but not Max, is a substrate for protein kinase-A (PK-A)-dependent phosphorylation. TEF-1 is phosphorylated by PK-A at residue serine-102. This post-translational modification of TEF-1 repressed its DNA-binding activity, but not its ability to interact with the Max protein. Replacement of serine-102 in TEF-1 by a neutral or a charged amino acid did not abolish its DNA-binding ability, suggesting that changing a charge at the 102 amino-acid position of TEF-1 was not sufficient to inhibit its DNA-binding activity. We also show that PK-A response of the alpha-MHC gene is stimulated by the presence of wild-type TEF-1 but not by mutant TEF-1 having serine-102 replaced by alanine, suggesting that phosphorylation at this residue accounts for the cAMP/PK-A response of the gene. Thus, these data demonstrate that TEF-1 is a direct target of cAMP/PK-A signaling in cardiac myocytes.
Figures
Figure 1
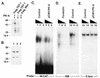
PK-A phosphorylation of TEF-1 inhibits its DNA-binding activity, but not its ability to interact with Max. (A) GST, GST-Max and GST-TEF-1 proteins (1 µg of each) were incubated with 10 U of the PK-A catalytic subunit in a kinase reaction buffer containing [γ-32P]ATP. Phosphorylation of the protein was visualized by SDS–PAGE followed by autoradiography. (B) EMSA was carried out using a 32P-labeled EM probe (as described in Materials and Methods) and GST-TEF-1 incubated with varying concentrations (10, 20 and 50 U) of the PK-A catalytic subunit and cold ATP. (C) In vitro translated [35S]methionine-labeled Max was incubated with glutathione–agarose beads bound to GST, GST-Max, GST-TEF-1 or PK-A-phosphorylated-GST-TEF-1 proteins. Proteins bound to beads were analyzed on SDS–polyacrylamide gels in the lanes indicated.
Figure 2
The PK-A phosphorylation site of TEF-1 is located within the first 113 aa of the protein. (A) Schematic diagram of TEF-1 deletion mutants and summary of their PK-A phosphorylation ability. (B, C) Different GST-TEF-1 proteins were subjected to PK-A phosphorylation using [γ-32P]ATP. Proteins were resolved by SDS–PAGE and visualized by Coomassie blue staining (B) and autoradiography (C) of the same gel. (D) EMSA was carried out with an M-CAT oligo as a labeled probe and increasing amounts (from 1 to 3 µg) of different GST-TEF-1 proteins as indicated at the top of the gel.
Figure 3
Serine-102 of TEF-1 is phosphorylated by PK-A. (A) Schematic diagram showing wild-type (wt) GST-TEF(1–113) peptide and two other mutants in which serine-102 was substituted by either alanine or aspartic acid. (B, C) Proteins were subjected to PK-A phosphorylation using [γ-32P]ATP and analyzed by SDS–PAGE followed by Coomassie blue staining (B) and autoradiography (C) of the same gel. (D) EMSA was carried out using an M-CAT oligo as a labeled probe and increasing amounts (from 1 to 3 µg) of the different proteins as indicated at the top of the gel. For the competition assay, a 100-fold excess of unlabeled M-CAT oligo was included in the EMSA-binding reaction.
Figure 4
Phosphorylation of TEF-1 by PK-A in vivo. (A) Sol8 cells in growth medium were transiently transfected with pCMVFlag.TEF-1 (lanes 1 and 2) or pCMVFlag.Max (lane 3) with and without PK-A expression plasmid. Eighteen hours after transfection, cells were transferred to differentiation medium, after which they were labeled with 32P (1 mCi/ml) for another 18 h. Cells were harvested, and Flag. recombinant proteins were immunoprecipitated from the cell lysate and resolved by SDS–PAGE. Proteins were transferred to PVDF membrane and visualized by autoradiography. (B) After 1 week, when 32P activity of the membrane was reduced, it was probed with anti-Flag antibody and subsequently analyzed by western blot analysis. (C, D, E) EMSA was performed using different probes (as described in Materials and Methods) and nuclear proteins (2, 4 and 8 µg) obtained from cells transfected with or without PK-A expression plasmid, or treated with 10 µM forskolin.
Figure 5
Involvement of TEF-1 in PK-A dependent activation of α-MHC gene expression. Primary cultures of fetal rat cardiac myocytes were cotransfected with 5 µg of the α-MHC/CAT reporter plasmid and varying amounts of expression vectors as shown in each panel. (A) Effect of different concentrations of pCMVTEF-1 and pCMVTEFmt-1 vectors on the expression of MHC/CAT reporter construct. (B) Effect of varying concentrations of PK-A expression vector alone, and when combined with TEF-1 expression vectors. CAT activity (mean ± S.E.) was normalized for β-galactosidase activity and expressed relative to the activity of pMHC/CAT in the vehicle-treated ventricular cells. Results are derived from 10–15 different cultures in which different plasmid preparations were used.
Figure 6
Identification of TEF-1 interactive domain with Max. (A) Schematic diagram of the different GST-TEF-1 mutants and a summary of their observed binding activity with Max. In the GST-TEF(1–113mt-1) and GST-TEF(1–113mt-2) peptides, serine-102 was changed to alanine and aspartic acid, respectively. (B) In the rabbit reticulocyte lysate pBS-MyoD, -myogenin and -Max plasmids were transcribed and translated with [35S]methionine. (C, D) The 35S-labeled Max (C), or MyoD (D) were incubated with GST or GST-TEF-1 fusion peptides on beads as indicated above each lane, and proteins bound to beads were analyzed on SDS–polyacrylamide gels.
Figure 7
The PK-A site (RRXS) of TEF-1 is not conserved among different TEF isoforms. Top panel: schematic diagram showing location of different regions of TEF-1. Lower panel: amino acids of the third helix of the TEA/ATTS DNA-binding domain and the PK-A site (underlined) of TEF-1 is shown and compared with other TEF isoforms. Four different human TEF isoforms, as a prototype for each group, are shown in bold letters.
Similar articles
- Transcription enhancer factor 1 interacts with a basic helix-loop-helix zipper protein, Max, for positive regulation of cardiac alpha-myosin heavy-chain gene expression.
Gupta MP, Amin CS, Gupta M, Hay N, Zak R. Gupta MP, et al. Mol Cell Biol. 1997 Jul;17(7):3924-36. doi: 10.1128/MCB.17.7.3924. Mol Cell Biol. 1997. PMID: 9199327 Free PMC article. - Sympathetic control of cardiac myosin heavy chain gene expression.
Gupta MP, Gupta M, Dizon E, Zak R. Gupta MP, et al. Mol Cell Biochem. 1996 Apr 12-26;157(1-2):117-24. doi: 10.1007/BF00227889. Mol Cell Biochem. 1996. PMID: 8739237 - Physical interaction between the MADS box of serum response factor and the TEA/ATTS DNA-binding domain of transcription enhancer factor-1.
Gupta M, Kogut P, Davis FJ, Belaguli NS, Schwartz RJ, Gupta MP. Gupta M, et al. J Biol Chem. 2001 Mar 30;276(13):10413-22. doi: 10.1074/jbc.M008625200. Epub 2001 Jan 2. J Biol Chem. 2001. PMID: 11136726 - Plant bZIP G-box binding factors. Modular structure and activation mechanisms.
Sibéril Y, Doireau P, Gantet P. Sibéril Y, et al. Eur J Biochem. 2001 Nov;268(22):5655-66. doi: 10.1046/j.0014-2956.2001.02552.x. Eur J Biochem. 2001. PMID: 11722549 Review. - AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins.
Leff T. Leff T. Biochem Soc Trans. 2003 Feb;31(Pt 1):224-7. doi: 10.1042/bst0310224. Biochem Soc Trans. 2003. PMID: 12546690 Review.
Cited by
- Tight junction protein ZO-2 modulates the nuclear accumulation of transcription factor TEAD.
Gallego-Gutiérrez H, González-González L, Ramírez-Martínez L, López-Bayghen E, González-Mariscal L. Gallego-Gutiérrez H, et al. Mol Biol Cell. 2021 Jul 15;32(15):1347-1358. doi: 10.1091/mbc.E20-07-0470. Epub 2021 May 19. Mol Biol Cell. 2021. PMID: 34010016 Free PMC article. - Regulation of TEAD Transcription Factors in Cancer Biology.
Huh HD, Kim DH, Jeong HS, Park HW. Huh HD, et al. Cells. 2019 Jun 17;8(6):600. doi: 10.3390/cells8060600. Cells. 2019. PMID: 31212916 Free PMC article. Review. - Alternative requirements for Vestigial, Scalloped, and Dmef2 during muscle differentiation in Drosophila melanogaster.
Deng H, Hughes SC, Bell JB, Simmonds AJ. Deng H, et al. Mol Biol Cell. 2009 Jan;20(1):256-69. doi: 10.1091/mbc.e08-03-0288. Epub 2008 Nov 5. Mol Biol Cell. 2009. PMID: 18987343 Free PMC article. - Prenatal exposure to dietary fat induces changes in the transcriptional factors, TEF and YAP, which may stimulate differentiation of peptide neurons in rat hypothalamus.
Poon K, Mandava S, Chen K, Barson JR, Buschlen S, Leibowitz SF. Poon K, et al. PLoS One. 2013 Oct 11;8(10):e77668. doi: 10.1371/journal.pone.0077668. eCollection 2013. PLoS One. 2013. PMID: 24147051 Free PMC article. - A TEF-1-element is required for activation of the promoter of pseudorabies virus glycoprotein X gene by IE180.
Ou CJ, Wong ML, Chang TJ. Ou CJ, et al. Virus Genes. 2002 Dec;25(3):241-53. doi: 10.1023/a:1020915706724. Virus Genes. 2002. PMID: 12881636
References
- Winter B., Braun,T. and Arnold,H.H. (1993) J. Biol. Chem., 268, 9869–9878. - PubMed
- Despande A.K. and Siddiqui,M.A.Q. (1976) Nature, 263, 588–591. - PubMed
- Zhou Q.Y., Quaif,C.J. and Palmiter,R.D. (1995) Nature, 374, 640–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous