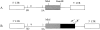Functional coexpression of HSV-1 thymidine kinase and green fluorescent protein: implications for noninvasive imaging of transgene expression - PubMed (original) (raw)
Functional coexpression of HSV-1 thymidine kinase and green fluorescent protein: implications for noninvasive imaging of transgene expression
A Jacobs et al. Neoplasia. 1999 Jun.
Abstract
Current gene therapy technology is limited by the paucity of methodology for determining the location and magnitude of therapeutic transgene expression in vivo. We describe and validate a paradigm for monitoring therapeutic transgene expression by noninvasive imaging of the herpes simplex virus type 1 thymidine kinase (HSV-1-tk) marker gene expression. To test proportional coexpression of therapeutic and marker genes, a model fusion gene comprising green fluorescent protein (gfp) and HSV-1-tk genes was generated (tkgfp gene) and assessed for the functional coexpression of the gene product, TKGFP fusion protein, in rat 9L gliosarcoma, RG2 glioma, and W256 carcinoma cells. Analysis of the TKGFP protein demonstrated that it can serve as a therapeutic gene by rendering tkgfp transduced cells sensitive to ganciclovir or as a screening marker useful for identifying transduced cells by fluorescence microscopy or fluorescence-activated cell sorting (FACS). TK and GFP activities in the TKGFP fusion protein were similar to corresponding wild-type proteins and accumulation of the HSV-1-tk-specific radiolabeled substrate, 2'-fluoro-2'-deoxy-1beta-D-arabinofuranosyl-5-iodo-uracil (FIAU), in stability transduced clones correlated with gfp-fluorescence intensity over a wide range of expression levels. The tkgfp fusion gene itself may be useful in developing novel cancer gene therapy approaches. Valuable information about the efficiency of gene transfer and expression could be obtained by non-invasive imaging of tkgfp expression with FIAU and clinical imaging devices (gamma camera, positron-emission tomography [PET], single photon emission computed tomography [SPECT]), and/or direct visualization of gfp expression in situ by fluorescence microscopy or endoscopy.
Figures
Figure 1
Structure and elements of TK and GFP expressing plasmids pTKGFP, pHSV-TK (HSV-1 amplicon plasmid as positive control for TK function) and pHSV-GFP (HSV-1 amplicon plasmid as positive control for GFP function). All genes of interest, tkgfp, native HSV-1-tk, and native gfp, respectively, are cloned under control of the CMV immediate early 1 promoter (pCMV). Further structural elements comprise a neomycin resistance gene (neoR) under control of an SV40 promoter. Controls contain also the HSV-1 origin of DNA replication (oriS) and the HSV-1 DNA cleavage/packaging signal (pac), which would allow packaging of these amplicon plamids into HSV-1 virions.
Figure 2
Construction of the SFG-TKGFP retroviral vector. The vector MoT (A) was used to produce the SFG-TKGFP vector (B) from pTKGFP (see Figure 1).
Figure 3
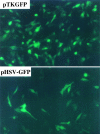
GFP expression in proliferating rat 9L gliosarcoma cells transfected with pTKGFP or pHSV-GFP. Comparable intensities of gfp fluorescence were identified in 9L cells 24 hours after transfection for both the pTKGFP expression plasmid and the pHSV-GFP amplicon plasmid (positive control).
Figure 4
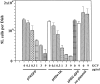
Ganciclovir dose-dependent killing of proliferating rat 9L gliosarcoma cells in culture. 9L cells (600,000 per 35-mm well; DAY zero) were transfected with 1 µg DNA of each pTKGFP, pHSV-TK (positive control), pHSV-GFP, or no plasmid (negative control) by using 6 µl lipofectamine. One day after transfection, GCV (0, 0.1, 0.3, 1, 3, and 9 µg/mL medium) was added to culture plates. Four days later surviving cells were trypsinized and counted. Bars represent mean values and SD from triplicate experiment.
Figure 5
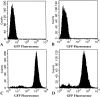
FACS analysis of gfp gene expression in single cells-derived clones of RG2GFPTK + (C) and W256GFPTK + (D) cells. Parental RG2 cells (A) and W256 cells (B) were used as controls.
Figure 6
Fusion-gene-mediated coexpression of the HSV-1-tk and gfp genes in culture. Gfp gene expression (mean GFP fluorscence/cell) was assessed by FACS analysis in RG2STLEO (A) and W256STLEO (B) transduced single cell clones and plotted against measurements of the HSV-1-tk gene expression (FIAU/TdR accumulation ratio). The relationship between the two measurements was defined by regression analysis. The same relationship was observed when the RG2STLEO and W256STLEO data sets were combined (C).
Figure 7
Western blot analysis of the TKGFP fusion protein. 4 µg of plasmid DNA of each, pTKGFP, pHSV-GFP, and pHSV-TK, respectively, were transfected into 9L cells. Monolayers were harvested 24 hours after transfection, resuspended, lysed, boiled, and proteins electrophoresed over night. After protein transfer and blocking of unspecific binding, the membrane was incubated with primary antibodies (1°; anti-TK 1:1000 and anti-GFP 1:1000) and, after washing, with horseradish peroxidase-conjugated horse anti-rabbit (1:4000) immunoglobulin, then washed and developed. As indicated, the primary anti-TK antibody recognizes TK at approximately 46 kD (third lane from left), and the primary GFP antibody recognizes the GFP at approximately 27 kD (fifth lane from left). Both antibodies recognize the TKGFP fusion protein at greater than 66 kD (first and fourth lane from left).
References
- Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. - PubMed
- Ezzeddine ZD, Martuza RL, Platika D, Short MP, Malick A, Choi B, Breakefield XO. Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biologist. 1991;3:608–614. - PubMed
- Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producing cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. - PubMed
- Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. - PubMed
- Paillard F. Suicide genes against brain tumors. Commentary. Hum Gene Ther. 1998;9:3–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA069246/CA/NCI NIH HHS/United States
- P01 CA059350/CA/NCI NIH HHS/United States
- R01 CA76117/CA/NCI NIH HHS/United States
- CA69246/CA/NCI NIH HHS/United States
- R01 CA59350/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources