Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors - PubMed (original) (raw)
Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors
G M Gillespie et al. J Virol. 2000 Sep.
Abstract
Human cytomegalovirus (HCMV) infection is largely asymptomatic in the immunocompetent host, but remains a major cause of morbidity in immunosuppressed individuals. Using the recently described technique of staining antigen-specific CD8(+) T cells with peptide-HLA tetrameric complexes, we have demonstrated high levels of antigen-specific cells specific for HCMV peptides and show that this may exceed 4% of CD8(+) T cells in immunocompetent donors. Moreover, by staining with tetramers in combination with antibodies to cell surface markers and intracellular cytokines, we demonstrate functional heterogeneity of HCMV-specific populations. A substantial proportion of these are effector cytotoxic T lymphocytes, as demonstrated by their ability to lyse peptide-pulsed targets in "fresh" killing assays. These data suggest that the immune response to HCMV is periodically boosted by a low level of HCMV replication and that sustained immunological surveillance contributes to the maintenance of host-pathogen homeostasis. These observations should improve our understanding of the immunobiology of persistent viral infection.
Figures
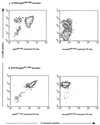
FIG. 1
Staining of CD8+ T-lymphocyte cultures with HCMV-specific HLA-peptide tetramers. Staining of CD8+ T-lymphocyte cultures with the tetrameric complex HLA-A*0201/pp65495–503. (a) A polyclonal CD8+ T-lymphocyte line specific for HCMV pp65495–503 is stained with a high degree of fluorescence by the tetramer. A control HLA-A*0201-restricted CTL line generated against influenza virus matrix peptide 58–66 does not show staining with this tetramer. (b) Tetramer HLA-B*0702/pp65417–426 binds specifically to a CTL clone grown on the pp65417–426 peptide, but fails to recognize a nonspecific HLA-B*0702-restricted CTL clone.
FIG. 2
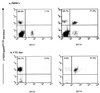
Representative data of tetramer staining of PBMCs from HCMV-seropositive and -seronegative donors. PBMCs from HCMV-seropositive and -seronegative donors were tested for their ability to bind the tetrameric complexes used in this investigation. Representative dot plots where tetrameric staining is represented along the x axis and CD8 staining is represented along the y axis are summarized. Both HLA-B*0702/pp65417–426 and HLA-A*0201/pp65495–503 tetramers display their ability to stain HCMV-specific CD8+ T lymphocytes in seropositive donors, yet display minimal binding to CD8+ T cells from HCMV-seronegative individuals. Plots a to c represent HLA-A*0201+ CMV-seropositive donors, and these samples were stained with A*0201/pp65495–503 tetrameric complexes. Plots d to f are representative HLA-B*0702+ CMV-seropositive donors, and these samples were stained with the B*0702/pp65417–426 tetramer. Plots g and h are HLA-A*0201+ CMV-seronegative donors which were stained with the A*0201/pp65495–503 tetramer. Plot i is an HLA-B*0702+ CMV-seronegative sample which was stained with the B*0702/pp65417–426 tetramer.
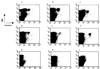
FIG. 3
Dual expression of CD45RA and CD45RO isoforms on tetramer-reactive CD8+ T lymphocytes from donor 13. Four-color FACS analysis, using a tetrameric complex in conjunction with anti-CD8, anti-CD45RA and anti-CD45RO MAbs, was performed with freshly isolated PBMCs from donor 13 in an attempt to estimate the proportion of cells which were positive for both the RA and RO isoforms of CD45. CD8+ tetramer-positive and CD8+ tetramer-negative cells were gated, and the percentages of cells coexpressing CD45RA and CD45RO within these subsets were estimated.
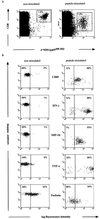
FIG. 4
Intracellular cytokine and chemokine in tetramer-reactive CD8+ cells. Freshly isolated PBMCs from donor 16 were permeabilized and stained with MAbs to CD69, IFN-γ, MIP-1β, TNF-α, and perforin either immediately following isolation or 6 h postactivation with cognate peptide. Both nonstimulated and peptide-stimulated CD8+ tetramer-positive cells were gated separately (a), and the proportions of cells within the given subsets expressing cytokines and chemokines (b) are summarized.
FIG. 5
Fresh cytotoxicity of PBMC fractions from HCMV-positive donors. Autologous 51Cr-labelled L-BCLs from donors 13 (a) and 17 (b) were incubated with antigenic peptide at a final peptide concentration of 5 μM and were used as targets in a 15-h 51Cr-release assay. Freshly isolated PBMCs were used as a source of effector cells. Cytolytic activity was assayed at various E:T ratios.
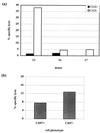
FIG. 6
(a) Cytotoxicity of CD28− and CD28+ CD8+ subsets. HLA-A*0201/pp65495–503-specific CD8+ T lymphocytes were sorted directly from PBMCs of donor 13 on the basis of the presence or absence of CD28 expression. Bulk CD8+ populations of CD28− and CD28+ CD8+ T cells were sorted by FACS from donors 16 and 17. Sorted populations were incubated with peptide-pulsed L-BCL targets, and cytotoxicity was assayed after 15 h. The E:T ratios were 0.5:1 for donor 13 and 2:1 for donors 16 and 17. The final peptide concentration was 5 μM. The percentage of specific lysis represents CTL lysis of pulsed targets − lysis of nonpulsed targets. (b) Cytotoxicity of CD57− and CD57+ CD8+ subsets. PBMCs from donor 13 were stained with A*0201/pp65495–503 tetrameric complex in conjunction with anti-CD8 and anti-CD57 MAbs and CD8+ tetramer-positive populations were sorted by FACS on the basis of the presence or absence of CD57. Sorted populations were incubated with peptide-pulsed L-BCL targets, and cytotoxicity was assayed after 15 h. The E:T ratio was 0.5:1.
FIG. 7
Staining of HLA-A*0201/pp65495–503-specific CTLs from donor 13 with a MAb specific for TCRBV14. Freshly isolated PBMCs and 12-day-old CTL lines were costained with anti-CD8-Tricolor, anti-TCR-FITC, and PE-conjugated HLA-A*0201/pp65495–503 tetramer. CD8+ lymphocytes were gated, and the proportions of tetramer-reactive cells expressing TCRBV14 both in fresh PBMCs (a) and the CTL line (b) were assessed. Control antibodies included anti-BV16 and anti-BV17 TCR MAbs.
FIG. 8
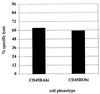
Cytolytic ability of sorted CD45ROhigh and CD45ROhigh tetramer-reactive cells propagated in vitro. PBMCs from donor 13 were stained with tetrameric complex, anti-CD8 MAb, and anti-CD45RA and anti-CD45RO antibodies, and populations of tetramer-reactive CD45RAhigh and CD45ROhigh cells were sorted by FACS and cultured in the presence of peptide-pulsed autologous L-BCLs. Their ability to lyse peptide-pulsed targets by a standard 51Cr-release assay was assessed following 14 days of culture. Both populations lysed target cells with similar efficiencies at E:T ratios of 10:1.
FIG. 9
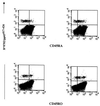
Segregation of tetramer-reactive cells expressing low, medium, and high levels of CD45RA and CD45RO isoforms. PBMCs were triple stained with anti-CD8 MAb, tetrameric complex, and either CD45RA or CD45RO isoform antibodies. CD8high populations were gated, and the percentages of tetramer-reactive cells expressing the CD45 isoforms were estimated. A typical dot plot of tetrameric staining versus CD45RA and CD45RO antigen expression from donor 17 is summarized.
Similar articles
- HCMV triggers frequent and persistent UL40-specific unconventional HLA-E-restricted CD8 T-cell responses with potential autologous and allogeneic peptide recognition.
Jouand N, Bressollette-Bodin C, Gérard N, Giral M, Guérif P, Rodallec A, Oger R, Parrot T, Allard M, Cesbron-Gautier A, Gervois N, Charreau B. Jouand N, et al. PLoS Pathog. 2018 Apr 30;14(4):e1007041. doi: 10.1371/journal.ppat.1007041. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29709038 Free PMC article. - Identification of HLA-A*2402-restricted HCMV immediate early-1 (IE-1) epitopes as targets for CD8+ HCMV-specific cytotoxic T lymphocytes.
Lim JB, Kim HO, Jeong SH, Ha JE, Jang S, Lee SG, Lee K, Stroncek D. Lim JB, et al. J Transl Med. 2009 Aug 23;7:72. doi: 10.1186/1479-5876-7-72. J Transl Med. 2009. PMID: 19698161 Free PMC article. - The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL.
Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. Wills MR, et al. J Virol. 1996 Nov;70(11):7569-79. doi: 10.1128/JVI.70.11.7569-7579.1996. J Virol. 1996. PMID: 8892876 Free PMC article. - Molecular characterization of HCMV-specific immune responses: Parallels between CD8(+) T cells, CD4(+) T cells, and NK cells.
Vieira Braga FA, Hertoghs KM, van Lier RA, van Gisbergen KP. Vieira Braga FA, et al. Eur J Immunol. 2015 Sep;45(9):2433-45. doi: 10.1002/eji.201545495. Epub 2015 Aug 24. Eur J Immunol. 2015. PMID: 26228786 Review. - Adoptive immunotherapy of HCMV infection.
Kapp M, Tan SM, Einsele H, Grigoleit G. Kapp M, et al. Cytotherapy. 2007;9(8):699-711. doi: 10.1080/14653240701656046. Epub 2007 Oct 4. Cytotherapy. 2007. PMID: 17917875 Review.
Cited by
- CD8(+) T-cell effector function and transcriptional regulation during HIV pathogenesis.
Demers KR, Reuter MA, Betts MR. Demers KR, et al. Immunol Rev. 2013 Jul;254(1):190-206. doi: 10.1111/imr.12069. Immunol Rev. 2013. PMID: 23772621 Free PMC article. Review. - Human cytomegalovirus miR-UL112-1 promotes the down-regulation of viral immediate early-gene expression during latency to prevent T-cell recognition of latently infected cells.
Lau B, Poole E, Van Damme E, Bunkens L, Sowash M, King H, Murphy E, Wills M, Van Loock M, Sinclair J. Lau B, et al. J Gen Virol. 2016 Sep;97(9):2387-2398. doi: 10.1099/jgv.0.000546. Epub 2016 Jul 13. J Gen Virol. 2016. PMID: 27411311 Free PMC article. - Reconstitution of HLA-A*2402-restricted cytomegalovirus-specific T-cells following stem cell transplantation.
Gondo H, Himeji D, Kamezaki K, Numata A, Tanimoto T, Takase K, Aoki K, Henzan H, Nagafuji K, Miyamoto T, Ishikawa F, Shimoda K, Inaba S, Tsukamoto H, Horiuchi T, Nakashima H, Otsuka T, Kato K, Kuroiwa M, Higuchi M, Shibuya T, Kamimura T, Kuzushima K, Tsurumi T, Kanda Y, Harada M. Gondo H, et al. Int J Hematol. 2004 Dec;80(5):441-8. doi: 10.1532/ijh97.04109. Int J Hematol. 2004. PMID: 15646657 - Divergent memory responses driven by adenoviral vectors are impacted by epitope competition.
Colston JM, Hutchings C, Chinnakannan S, Highton A, Perez-Shibayama C, Ludewig B, Klenerman P. Colston JM, et al. Eur J Immunol. 2019 Sep;49(9):1356-1363. doi: 10.1002/eji.201948143. Epub 2019 May 29. Eur J Immunol. 2019. PMID: 31106398 Free PMC article. - Age-related appearance of a CMV-specific high-avidity CD8+ T cell clonotype which does not occur in young adults.
Schwanninger A, Weinberger B, Weiskopf D, Herndler-Brandstetter D, Reitinger S, Gassner C, Schennach H, Parson W, Würzner R, Grubeck-Loebenstein B. Schwanninger A, et al. Immun Ageing. 2008 Nov 12;5:14. doi: 10.1186/1742-4933-5-14. Immun Ageing. 2008. PMID: 19014475 Free PMC article.
References
- Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
- Borysiewicz L K, Graham S, Hickling J K, Mason P D, Sissons J G. Human cytomegalovirus-specific cytotoxic T cells: their precursor frequency and stage specificity. Eur J Immunol. 1988;18:269–275. - PubMed
- Borysiewicz L K, Morris S, Page J D, Sissons J G. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur J Immunol. 1983;13:804–809. - PubMed
- Britt W J, Alford C A. Cytomegalovirus. 3rd ed. New York, N.Y: Lippincott-Raven; 1996.
- Bunce M, O'Neill C M, Barnardo M C, Krausa P, Browning M J, Morris P J, Welsh K I. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials