A monomeric GTPase-negative MxA mutant with antiviral activity - PubMed (original) (raw)
A monomeric GTPase-negative MxA mutant with antiviral activity
C Janzen et al. J Virol. 2000 Sep.
Abstract
MxA is a large, interferon-induced GTPase with antiviral activity against RNA viruses. It forms large oligomers, but whether oligomerization and GTPase activity are important for antiviral function is not known. The mutant protein MxA(L612K) carries a lysine-for-leucine substitution at position 612 and fails to form oligomers. Here we show that monomeric MxA(L612K) lacks detectable GTPase activity but is capable of inhibiting Thogoto virus in transiently transfected Vero cells or in a Thogoto virus minireplicon system. Likewise, MxA(L612K) inhibited vesicular stomatitis virus multiplication. These findings indicate that MxA monomers are antivirally active and suggest that GTP hydrolysis may not be required for antiviral activity. MxA(L612K) is rapidly degraded in cells, whereas wild-type MxA is stable. We propose that high-molecular-weight MxA oligomers represent a stable intracellular pool from which active MxA monomers are recruited.
Figures
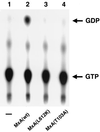
FIG. 1
MxA(L612K) lacks detectable GTPase activity. GTP hydrolysis of mutant MxA(L612K) protein was compared with that of wild-type MxA [MxA(wt)] or inactive mutant MxA(T103A) in a standard GTPase assay. Lane 1 is a control without protein in the reaction mixture.
FIG. 2
MxA(L612K) is antivirally active. (A) Vero cells transfected with cDNAs encoding either wild-type MxA [MxA(wt)] or mutant protein MxA(L612K) or MxA(T103A) were infected with 50 PFU of THOV per cell. Cells were fixed 9 h later, and MxA proteins or viral proteins were detected by double immunofluorescence using a monoclonal antibody directed against MxA and a polyclonal antiserum directed against THOV antigens. (B) Quantitative analysis of infection experiments with THOV and VSV. The percentage of infected cells expressing either wild-type MxA [MxA(wt)], MxA(L612K), or MxA(T103A) is shown. Cells were infected with 10 PFU of VSV serotype Indiana per cell (15).
FIG. 3
MxA(L612K) inhibits reporter gene expression in a THOV minireplicon system. COS-1 cells were transfected with T7 promoter constructs coding for the components of a THOV minireplicon system as previously described (29). In this system, synthesis of CAT protein reflects the activity of the reconstituted vRNPs (30). Wild-type or mutant MxA was expressed under the control of the T7 promoter using increasing amounts of expression plasmid pBS-T7/MxA, pBS-T7/MxA(L612K), or pBS-T7/MxA(T103A). (A) CAT protein concentration as determined by a colorimetric immunoassay (Boehringer Mannheim). The amounts of CAT and luciferase activity were determined in the cell lysates. Luciferase activity was used to normalize CAT expression, and the ratio of CAT protein concentration to luciferase activity (CAT/Luc ratio) was calculated as described previously (29). The CAT/luciferase ratio of experiments without MxA were set at 1. (B) Expression of MxA proteins. Aliquots of the cell lysates (15 μg of protein per lane) were analyzed by Western blotting using a polyclonal antiserum directed against MxA.
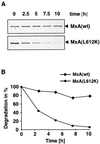
FIG. 4
MxA(L612K) is rapidly degraded. COS-1 cells were transfected with expression plasmids coding for either MxA or MxA(L612K). Cells were then labeled for 2 h with 35 μCi of [35S]methionine per ml and chased for 2.5, 5, 7.5, and 10 h. (A) Wild-type and mutant MxA proteins were immunoprecipitated from cell lysates using a monoclonal antibody directed against MxA and analyzed by polyacrylamide gel electrophoresis and autoradiography. (B) PhosphoImager analysis of immunoprecipitated proteins. MxA(wt), wild-type MxA.
Similar articles
- Dominant-negative mutants of human MxA protein: domains in the carboxy-terminal moiety are important for oligomerization and antiviral activity.
Ponten A, Sick C, Weeber M, Haller O, Kochs G. Ponten A, et al. J Virol. 1997 Apr;71(4):2591-9. doi: 10.1128/JVI.71.4.2591-2599.1997. J Virol. 1997. PMID: 9060610 Free PMC article. - Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus.
Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O. Frese M, et al. J Virol. 1995 Jun;69(6):3904-9. doi: 10.1128/JVI.69.6.3904-3909.1995. J Virol. 1995. PMID: 7745744 Free PMC article. - GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae).
Kochs G, Haller O. Kochs G, et al. J Biol Chem. 1999 Feb 12;274(7):4370-6. doi: 10.1074/jbc.274.7.4370. J Biol Chem. 1999. PMID: 9933640 - [Alpha interferon, antiviral proteins and their value in clinical medicine].
Chieux V, Hober D, Chehadeh W, Wattré P. Chieux V, et al. Ann Biol Clin (Paris). 1999 Nov-Dec;57(6):659-66. Ann Biol Clin (Paris). 1999. PMID: 10572214 Review. French. - Mx proteins: GTPases involved in the interferon-induced antiviral state.
Pavlovic J, Schröder A, Blank A, Pitossi F, Staeheli P. Pavlovic J, et al. Ciba Found Symp. 1993;176:233-43; discussion 243-7. doi: 10.1002/9780470514450.ch15. Ciba Found Symp. 1993. PMID: 7507812 Review.
Cited by
- Mouse guanylate-binding proteins of the chromosome 3 cluster do not mediate antiviral activity in vitro or in mouse models of infection.
Tessema MB, Feng S, Enosi Tuipulotu D, Farrukee R, Ngo C, Gago da Graça C, Yamomoto M, Utzschneider DT, Brooks AG, Londrigan SL, Man SM, Reading PC. Tessema MB, et al. Commun Biol. 2024 Aug 25;7(1):1050. doi: 10.1038/s42003-024-06748-8. Commun Biol. 2024. PMID: 39183326 Free PMC article. - Oligomerization and GTP-binding Requirements of MxA for Viral Target Recognition and Antiviral Activity against Influenza A Virus.
Nigg PE, Pavlovic J. Nigg PE, et al. J Biol Chem. 2015 Dec 11;290(50):29893-906. doi: 10.1074/jbc.M115.681494. Epub 2015 Oct 27. J Biol Chem. 2015. PMID: 26507657 Free PMC article. - The Antiviral Activity of Equine Mx1 against Thogoto Virus Is Determined by the Molecular Structure of Its Viral Specificity Region.
Wagner V, Sabachvili M, Bendl E, Fuchs J, Kochs G. Wagner V, et al. J Virol. 2023 Feb 28;97(2):e0193822. doi: 10.1128/jvi.01938-22. Epub 2023 Feb 7. J Virol. 2023. PMID: 36749070 Free PMC article. - Inhibition of a large double-stranded DNA virus by MxA protein.
Netherton CL, Simpson J, Haller O, Wileman TE, Takamatsu HH, Monaghan P, Taylor G. Netherton CL, et al. J Virol. 2009 Mar;83(5):2310-20. doi: 10.1128/JVI.00781-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109387 Free PMC article. - Role of interferons in the treatment of severe acute respiratory syndrome.
Cinatl J Jr, Michaelis M, Scholz M, Doerr HW. Cinatl J Jr, et al. Expert Opin Biol Ther. 2004 Jun;4(6):827-36. doi: 10.1517/14712598.4.6.827. Expert Opin Biol Ther. 2004. PMID: 15174965 Free PMC article. Review.
References
- Albanese M, Bruno-Smiraglia C, Di Di Cuonza G, Lavagnino A, Srihongse S. Isolation of Thogoto virus from Rhipicephalus bursa ticks in western Sicily. Acta Virol. 1972;16:267. - PubMed
- Di Paolo C. Role of the carboxy-terminal domain of MxA protein in antiviral activity and protein-protein interactions. Ph.D. thesis. Zurich, Switzerland: University of Zurich; 1998.
- Di Paolo C, Hefti H P, Meli M, Landis H, Pavlovic J. Intramolecular backfolding of the carboxyl-terminal end of MxA protein is a prerequisite for its oligomerization. J Biol Chem. 1999;274:32071–32078. - PubMed
- Flohr F, Schneider-Schaulies S, Haller O, Kochs G. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 1999;463:24–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources