F-actin is concentrated in nonrelease domains at frog neuromuscular junctions - PubMed (original) (raw)
F-actin is concentrated in nonrelease domains at frog neuromuscular junctions
A Dunaevsky et al. J Neurosci. 2000.
Abstract
To gain insight into the role of F-actin in the organization of synaptic vesicles at release sites, we examined the synaptic distribution of F-actin by using a unique synaptic preparation of frog target-deprived nerve terminals. In this preparation, imaging of the synaptic site was unobstructed by the muscle fiber cytoskeleton, allowing for the examination of hundreds of synaptic sites in their entirety in whole mounts. At target-deprived synaptic sites F-actin was distributed in a ladder-like pattern and was colocalized with beta-fodrin. Surprisingly, F-actin stain, which we localized to the nerve terminal itself, did not overlap a synaptic vesicle marker, suggesting that it was concentrated in nonrelease domains of nerve terminals between clusters of synaptic vesicles. These findings suggest that the majority of the presynaptic F-actin is not involved in tethering synaptic vesicles. Instead, the strategic presynaptic positioning of this cytoskeletal meshwork in nonrelease domains of the nerve terminal suggests alternate functions such as restricting synaptic vesicles to release domains, recycling synaptic vesicles, or stabilizing the nerve terminal.
Figures
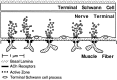
Fig. 1.
Longitudinal view through a region of a frog neuromuscular junction. The nerve terminal, capped by a terminal Schwann cell, is separated from the muscle membrane by the synaptic basal lamina. Several release sites in the nerve terminal are filled with synaptic vesicles and are aligned with junctional folds in the muscle membrane. Processes of the terminal Schwann cell encircle the nerve terminal in some nonrelease domains.
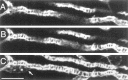
Fig. 2.
A target-deprived synaptic site is stained by phalloidin in a ladder-like pattern. A, B, Two confocal_z-_sections of a target-deprived nerve terminal stained with rhodamine-conjugated phalloidin to mark F-actin. C, A projection of a _z-_section series of 42 images. A terminal Schwann cell nucleus is marked with an arrow. Scale bar, 10 μm.
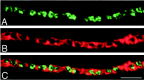
Fig. 3.
The majority of F-actin stain is external to synaptic vesicle clusters. A target-deprived synaptic site is stained with SV2 (A) to mark synaptic vesicle clusters and with phalloidin (B) to mark F-actin.C, The merged pseudocolor images reveal that the bands of actin are located between synaptic vesicle clusters rather than at the release sites. Scale bar, 5 μm.
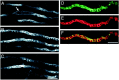
Fig. 4.
β-Fodrin stain is distributed in a ladder-like pattern at target-deprived synaptic sites and colocalizes with F-actin stain. A–C, Left, Three confocal_z-sections through target-deprived synaptic sites are stained with an antibody directed against β-fodrin. An_arrow marks a nucleus of a terminal Schwann cell. Scale bar, 10 μm. Right, Pseudocolor images of a target-deprived synaptic site stained with an antibody directed against β-fodrin (D) and phalloidin (E). F, A merged image of_D_ and E. Scale bar, 5 μm.
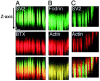
Fig. 5.
Bands of F-actin and β-fodrin stain do not persist after denervation of target-deprived synaptic sites. Innervated (A) or 2 week denervated (B, C) synaptic sites are marked with peanut agglutinin (PNA) and stained for actin microfilaments or β-fodrin. Scale bar, 5 μm.
Fig. 6.
Terminal Schwann cell stain is not colocalized with bands of F-actin stain. Target-deprived synaptic sites were double-labeled with markers of terminal Schwann cells, either SC-1 or 2A12, and phalloidin. The terminal Schwann cell stain was rarely banded and did not overlap the bands of phalloidin stain. Scale bar, 5 μm.
Fig. 7.
The distribution of F-actin at a synaptic site is coextensive with that of synaptic vesicles. Each_z-series of confocal images was resampled along a specified line to yield a longitudinal view of a synaptic site. The view from top to bottom(arrow) shows a section through the nerve terminal toward the presynaptic membrane and synaptic cleft. Each_column of three images (A–C) shows reconstructed line scans of a synaptic site stained both with an antibody to either SV2 or β-fodrin (in green) and markers of either acetylcholine receptors (BTX) or F-actin (in red). The _bottom panels_represent overlays of images from the pairs of stain. Markers of synaptic structures found in distinct locations (A) were resolved in the _z_-axis by this method. F-actin is coextensive with a synaptic vesicle marker (C) and overlaps completely with β-fodrin (B); co-ordinates: _x_-axis, 3 μm;_z_-axis, 0.5 μm.
Similar articles
- The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1.
Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. Hirokawa N, et al. J Cell Biol. 1989 Jan;108(1):111-26. doi: 10.1083/jcb.108.1.111. J Cell Biol. 1989. PMID: 2536030 Free PMC article. - The actin cytoskeleton and neurotransmitter release: an overview.
Doussau F, Augustine GJ. Doussau F, et al. Biochimie. 2000 Apr;82(4):353-63. doi: 10.1016/s0300-9084(00)00217-0. Biochimie. 2000. PMID: 10865123 Review. - Synaptic vesicle pools at the frog neuromuscular junction.
Richards DA, Guatimosim C, Rizzoli SO, Betz WJ. Richards DA, et al. Neuron. 2003 Jul 31;39(3):529-41. doi: 10.1016/s0896-6273(03)00405-7. Neuron. 2003. PMID: 12895425 - Actin in a supporting role.
Halpain S. Halpain S. Nat Neurosci. 2003 Feb;6(2):101-2. doi: 10.1038/nn0203-101. Nat Neurosci. 2003. PMID: 12555098 Review. No abstract available.
Cited by
- Hypothesis Relating the Structure, Biochemistry and Function of Active Zone Material Macromolecules at a Neuromuscular Junction.
Szule JA. Szule JA. Front Synaptic Neurosci. 2022 Jan 5;13:798225. doi: 10.3389/fnsyn.2021.798225. eCollection 2021. Front Synaptic Neurosci. 2022. PMID: 35069169 Free PMC article. - Actin- and dynamin-dependent maturation of bulk endocytosis restores neurotransmission following synaptic depletion.
Nguyen TH, Maucort G, Sullivan RK, Schenning M, Lavidis NA, McCluskey A, Robinson PJ, Meunier FA. Nguyen TH, et al. PLoS One. 2012;7(5):e36913. doi: 10.1371/journal.pone.0036913. Epub 2012 May 22. PLoS One. 2012. PMID: 22629340 Free PMC article. - Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction.
Richards DA, Rizzoli SO, Betz WJ. Richards DA, et al. J Physiol. 2004 May 15;557(Pt 1):77-91. doi: 10.1113/jphysiol.2004.062158. Epub 2004 Mar 5. J Physiol. 2004. PMID: 15004214 Free PMC article. - A Na,K-ATPase-Fodrin-Actin Membrane Cytoskeleton Complex is Required for Endothelial Fenestra Biogenesis.
Ju M, Ioannidou S, Munro P, Rämö O, Vihinen H, Jokitalo E, Shima DT. Ju M, et al. Cells. 2020 Jun 3;9(6):1387. doi: 10.3390/cells9061387. Cells. 2020. PMID: 32503129 Free PMC article. - Membrane compression by synaptic vesicle exocytosis triggers ultrafast endocytosis.
Ogunmowo TH, Jing H, Raychaudhuri S, Kusick GF, Imoto Y, Li S, Itoh K, Ma Y, Jafri H, Dalva MB, Chapman ER, Ha T, Watanabe S, Liu J. Ogunmowo TH, et al. Nat Commun. 2023 May 20;14(1):2888. doi: 10.1038/s41467-023-38595-2. Nat Commun. 2023. PMID: 37210439 Free PMC article.
References
- Astrow SH, Qiang H, Ko C-P. Perisynaptic Schwann cells at neuromuscular junctions revealed by a novel monoclonal antibody. J Neurocytol. 1999;27:667–681. - PubMed
- Beck KA, Nelson WJ. The spectrin-based membrane skeleton as a protein-sorting machine. Am J Physiol. 1996;270:C1263–C1270. - PubMed
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. - PubMed
- Bernstein BW, DeWitt M, Bamburg JR. Actin disassembles reversibly during electrically induced recycling of synaptic vesicles in cultured neurons. Mol Brain Res. 1998;53:236–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources