Dissociable neural responses in human reward systems - PubMed (original) (raw)
Dissociable neural responses in human reward systems
R Elliott et al. J Neurosci. 2000.
Abstract
Reward is one of the most important influences shaping behavior. Single-unit recording and lesion studies in experimental animals have implicated a number of regions in response to reinforcing stimuli, in particular regions of the extended limbic system and the ventral striatum. In this experiment, functional neuroimaging was used to assess neural response within human reward systems under different psychological contexts. Nine healthy volunteers were scanned using functional magnetic resonance imaging during the performance of a gambling task with financial rewards and penalties. We demonstrated neural sensitivity of midbrain and ventral striatal regions to financial rewards and hippocampal sensitivity to financial penalties. Furthermore, we show that neural responses in globus pallidus, thalamus, and subgenual cingulate were specific to high reward levels occurring in the context of increasing reward. Responses to both reward level in the context of increasing reward and penalty level in the context of increasing penalty were seen in caudate, insula, and ventral prefrontal cortex. These results demonstrate dissociable neural responses to rewards and penalties that are dependent on the psychological context in which they are experienced.
Figures
Fig. 1.
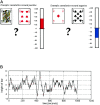
Cognitive activation paradigm.a shows the task that was used; the pairs of black and red cards to one side of the screen and the cumulative reward bar to the other. b shows the sequence of outcomes, corresponding to the actual rewards and penalties experienced, derived from the constrained binomial random walk function. This same function determined outcomes in all subjects, who consequently experienced the same sequence of rewards and penalties. The red asterisks mark the point at which blocks of rest were intersposed. These rest blocks were used to model low frequency drift in signal and were not involved in the subsequent regression analysis.
Fig. 2.
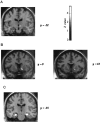
Responses to reward level. The figure shows SPMs of the t statistic (after transformation to a SPM{Z}) rendered onto a standard MRI template.a shows the activation in right midbrain positively associated with reward level (x = 9;y = −12; z = −3), above the substantia nigra and below the thalamus. b shows activation in right ventral striatum (x = 18;y = 9 or 12; z = −6) lateral to the nucleus accumbens. Both activations were significant at_p_ < 0.001. c shows activation of left hippocampus (x = −24; y = −18; z = −18; *p < 0.05) and right hippocampus (x = 33; y = −12; z = −21; p < 0.001) negatively associated with reward level.
Fig. 3.
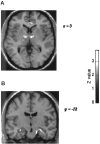
Responses to reward in the context of a winning streak. The transverse slice in a displays activations of right anteroventral thalamus (area VA; x = 6;y = −12; z = 0; *p < 0.05), bilateral globus pallidus (x = +15; y = −3;z = 6; p < 0.001), and subgenual cingulate (x = +9; _y_= −36; z = 3; p < 0.001) associated with reward occurring during a winning streak. These group activations appear somewhat spread because of the spatial smoothing necessary for intersubject averaging. Therefore it should be noted that we cannot be certain that the putative thalamus and global pallidus activations indeed represent regionally distinct neural responses, although this is how we have chosen to interpret them. _b_shows enhanced neural response in the bilateral hippocampus associated with the experience of high levels of penalty during a losing streak. It should be noted that the activation in the right hippocampal region is contaminated by the large blood vessel.
Fig. 4.
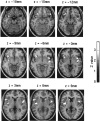
Responses to generic risk-taking processes. Activations of right (x = 51;y = 15; z = −12;p < 0.001) and left (x = −33;y = 15; z = −15; *p < 0.05) orbitofrontal cortex, right (x = 42; y = 9;z = −6; *p < 0.05) and left (x = −36; y = −6;z = 6; *p < 0.05) insula, and right (x = 21; y = 24;z = 0; *p < 0.05) and left (x = −9; y = 12;z = 12; p < 0.001) caudate associated with both reward during a winning streak and penalties during a losing streak. These activations may represent responses to risk-taking, independent of outcome.
Similar articles
- Context-dependent cortical activation in response to financial reward and penalty: an event-related fMRI study.
Akitsuki Y, Sugiura M, Watanabe J, Yamashita K, Sassa Y, Awata S, Matsuoka H, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Akitsuki Y, et al. Neuroimage. 2003 Aug;19(4):1674-85. doi: 10.1016/s1053-8119(03)00250-7. Neuroimage. 2003. PMID: 12948722 - Monkey brain activity modulated by reward preferences: a positron emission tomography study.
Obayashi S, Nagai Y, Suhara T, Okauchi T, Inaji M, Iriki A, Maeda J. Obayashi S, et al. Neurosci Res. 2009 Aug;64(4):421-8. doi: 10.1016/j.neures.2009.04.016. Epub 2009 May 3. Neurosci Res. 2009. PMID: 19416743 - Different neural systems adjust motor behavior in response to reward and punishment.
Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A. Wrase J, et al. Neuroimage. 2007 Jul 15;36(4):1253-62. doi: 10.1016/j.neuroimage.2007.04.001. Epub 2007 Apr 5. Neuroimage. 2007. PMID: 17521924 - Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.
Hollerman JR, Tremblay L, Schultz W. Hollerman JR, et al. Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review. - Distinct neurofunctional alterations during motivational and hedonic processing of natural and monetary rewards in depression - a neuroimaging meta-analysis.
Bore MC, Liu X, Gan X, Wang L, Xu T, Ferraro S, Li L, Zhou B, Zhang J, Vatansever D, Biswal B, Klugah-Brown B, Becker B. Bore MC, et al. Psychol Med. 2024 Mar;54(4):639-651. doi: 10.1017/S0033291723003410. Epub 2023 Nov 24. Psychol Med. 2024. PMID: 37997708 Review.
Cited by
- Positively valenced stimuli facilitate creative novel metaphoric processes by enhancing medial prefrontal cortical activation.
Subramaniam K, Beeman M, Faust M, Mashal N. Subramaniam K, et al. Front Psychol. 2013 Apr 26;4:211. doi: 10.3389/fpsyg.2013.00211. eCollection 2013. Front Psychol. 2013. PMID: 23637686 Free PMC article. - A time estimation task as a possible measure of emotions: difference depending on the nature of the stimulus used.
Gros A, Giroud M, Bejot Y, Rouaud O, Guillemin S, Aboa Eboulé C, Manera V, Daumas A, Lemesle Martin M. Gros A, et al. Front Behav Neurosci. 2015 Jun 11;9:143. doi: 10.3389/fnbeh.2015.00143. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26124711 Free PMC article. - The optimal calibration hypothesis: how life history modulates the brain's social pain network.
Chester DS, Pond RS Jr, Richman SB, Dewall CN. Chester DS, et al. Front Evol Neurosci. 2012 Jul 5;4:10. doi: 10.3389/fnevo.2012.00010. eCollection 2012. Front Evol Neurosci. 2012. PMID: 22783189 Free PMC article. - Abnormal neural responses to social exclusion in schizophrenia.
Gradin VB, Waiter G, Kumar P, Stickle C, Milders M, Matthews K, Reid I, Hall J, Steele JD. Gradin VB, et al. PLoS One. 2012;7(8):e42608. doi: 10.1371/journal.pone.0042608. Epub 2012 Aug 16. PLoS One. 2012. PMID: 22916139 Free PMC article. - Understanding the placebo effect: contributions from neuroimaging.
Lidstone SC, Stoessl AJ. Lidstone SC, et al. Mol Imaging Biol. 2007 Jul-Aug;9(4):176-85. doi: 10.1007/s11307-007-0086-3. Mol Imaging Biol. 2007. PMID: 17334853 Review.
References
- Alexander GE, DeLong M, Strick PE. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
- Aosaki T, Graybiel AM, Kimura M. Effects of nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkey. Science. 1994;265:412–415. - PubMed
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. - PubMed
- Arvanitogiannis A, Waraczynski M, Shizgal P. Effects of excitotoxic lesions of the basal forebrain on MFB self-stimulation. Physiol Behav. 1996;59:795–906. - PubMed
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources