Prefrontal-temporal circuitry for episodic encoding and subsequent memory - PubMed (original) (raw)
Prefrontal-temporal circuitry for episodic encoding and subsequent memory
B A Kirchhoff et al. J Neurosci. 2000.
Abstract
Humans encounter and form memories for multiple types of experiences that differ in content, novelty, and memorability. Critical for understanding memory is determining (1) how the brain supports the encoding of events with differing content and (2) whether neural regions that are sensitive to novelty also influence whether stimuli will be subsequently remembered. This event-related functional magnetic resonance imaging (fMRI) study crossed content (picture/word), novelty (novel/repeated), and subsequent memory (remembered/forgotten) to examine prefrontal and temporal lobe contributions to encoding. Results revealed three patterns of encoding-related activation in anatomically connected inferior prefrontal and lateral temporal structures that appeared to vary depending on whether visuospatial/visuo-object, phonological/lexical, or semantic attributes were processed. Event content also modulated medial temporal lobe activity; word encoding predominantly activated the left hemisphere, whereas picture encoding activated both hemispheres. Critically, in prefrontal and temporal regions that were modulated by novelty, the magnitude of encoding activation also predicted whether an event would be subsequently remembered. These results suggest that (1) regions that demonstrate a sensitivity to novelty may actively support encoding processes that impact subsequent explicit memory and (2) multiple content-dependent prefrontal-temporal circuits support event encoding. The similarities between prefrontal and lateral temporal encoding responses raise the possibility that prefrontal modulation of posterior cortical representations is central to encoding.
Figures
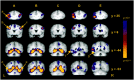
Fig. 1.
Inferior prefrontal, fusiform, and left lateral temporal encoding responses. Columns A–E, Activity during novel stimulus (novel > repeated pictures + words), novel picture (novel > repeated), picture versus word (novel picture > novel word), novel word (novel > repeated), and word versus picture (novel word > novel picture) encoding, respectively. Regions of interest were defined from the novel stimulus (a–e), novel word (f), and word versus picture (g) comparisons. a, Anterior LIPC: −37, 25, 3; BA 45/47. b, Posterior LIPC: −34, 6, 34; BA 6/44. c, Posterior RIPC: 43, 3, 37; BA 6/44. d, Left posterior fusiform: −28, −55, −9; BA 37. e, Right posterior fusiform: 34, −49, −12; BA 37. f, Left lateral temporal cortex: −56, −43, 3; BA 21/22. g, Left anterior fusiform: −40, −43, −6; BA 37. L, Left; R, right.
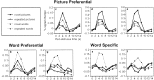
Fig. 2.
Percent signal change time courses from the inferior prefrontal, fusiform, and left lateral temporal regions of interest shown in Figure 1 and labeled a–g. The posterior RIPC (c) and bilateral posterior fusiform regions (d, e) demonstrated picture preferential encoding responses, the posterior LIPC (b) and left anterior fusiform (g) demonstrated word preferential encoding responses, and the anterior LIPC (a) and left lateral temporal cortex (f) demonstrated word specific encoding responses.
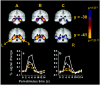
Fig. 3.
Parahippocampal/fusiform regions engaged in novel stimulus encoding. Columns A–C represent novel stimulus, novel picture, and novel word comparisons, respectively. To compare the percent signal change responses to novel pictures and novel words in similar regions in both hemispheres, we used the coordinates from the left parahippocampal/ fusiform ROI in the novel stimulus condition to grow a region in the right hemisphere. a(−21, −40, −9; BA 35/36/37) and b (21, −40, −9; BA 35/36/37) reflect the percent signal change time courses from these ROIs. Picture and word encoding activated overlapping regions of the posterior MTL. Novel pictures, solid white line with_white squares_; repeated pictures, dashed white line with white squares; novel words,solid yellow line with yellow squares; repeated words, dashed yellow line with yellow squares.
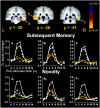
Fig. 4.
Medial temporal regions that were more active during the encoding of pictures that were subsequently remembered than during the encoding of pictures that were subsequently forgotten.a, 28, −30, −6; hippocampus. b, −28, −37, −9; BA35/36/37. c, 31, −40, −6; BA 35/36/37. Graphs a–c, Subsequent memory percent signal change time courses. Graphs a1_–c1, Novelty percent signal change time courses for the same ROIs depicted in a–c, respectively. Medial temporal regions that demonstrated picture subsequent memory effects also revealed picture and word novelty responses and trends for word subsequent memory effects. Graphs a–c, Remembered pictures, solid white line with_white squares; forgotten pictures, dashed white line with white squares; remembered words,solid yellow line with yellow squares; forgotten words, dashed yellow line with yellow squares. _Graphs a1_–c1, Novel pictures, solid white line with white squares; repeated pictures, _dashed white line_with white squares; novel words, solid yellow line with yellow squares; repeated words,dashed yellow line with yellow squares.
Similar articles
- Conceptual and perceptual novelty effects in human medial temporal cortex.
O'Kane G, Insler RZ, Wagner AD. O'Kane G, et al. Hippocampus. 2005;15(3):326-32. doi: 10.1002/hipo.20053. Hippocampus. 2005. PMID: 15490462 - Anterior hippocampus orchestrates successful encoding and retrieval of non-relational memory: an event-related fMRI study.
Kircher T, Weis S, Leube D, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Krach S. Kircher T, et al. Eur Arch Psychiatry Clin Neurosci. 2008 Sep;258(6):363-72. doi: 10.1007/s00406-008-0805-z. Epub 2008 Apr 24. Eur Arch Psychiatry Clin Neurosci. 2008. PMID: 18437279 Clinical Trial. - Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons.
Otten LJ, Henson RN, Rugg MD. Otten LJ, et al. Brain. 2001 Feb;124(Pt 2):399-412. doi: 10.1093/brain/124.2.399. Brain. 2001. PMID: 11157567 Clinical Trial. - Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: human declarative memory formation at the system level.
Fernández G, Tendolkar I. Fernández G, et al. Brain Res Bull. 2001 May 1;55(1):1-9. doi: 10.1016/s0361-9230(01)00494-4. Brain Res Bull. 2001. PMID: 11427332 Review. - Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex.
Ranganath C. Ranganath C. Neuroscience. 2006 Apr 28;139(1):277-89. doi: 10.1016/j.neuroscience.2005.06.092. Epub 2005 Dec 15. Neuroscience. 2006. PMID: 16343785 Review.
Cited by
- Neural substrates of successful working memory and long-term memory formation in a relational spatial memory task.
Bergmann HC, Daselaar SM, Fernández G, Kessels RP. Bergmann HC, et al. Cogn Process. 2016 Nov;17(4):377-387. doi: 10.1007/s10339-016-0772-7. Epub 2016 Jun 27. Cogn Process. 2016. PMID: 27350001 Free PMC article. - Current questions on space and time encoding.
Hasselmo ME, Stern CE. Hasselmo ME, et al. Hippocampus. 2015 Jun;25(6):744-52. doi: 10.1002/hipo.22454. Epub 2015 Apr 15. Hippocampus. 2015. PMID: 25786389 Free PMC article. Review. - Rapid microstructural plasticity in the cortical semantic network following a short language learning session.
Vukovic N, Hansen B, Lund TE, Jespersen S, Shtyrov Y. Vukovic N, et al. PLoS Biol. 2021 Jun 14;19(6):e3001290. doi: 10.1371/journal.pbio.3001290. eCollection 2021 Jun. PLoS Biol. 2021. PMID: 34125828 Free PMC article. - Listen, learn, like! Dorsolateral prefrontal cortex involved in the mere exposure effect in music.
Green AC, Bærentsen KB, Stødkilde-Jørgensen H, Roepstorff A, Vuust P. Green AC, et al. Neurol Res Int. 2012;2012:846270. doi: 10.1155/2012/846270. Epub 2012 Mar 28. Neurol Res Int. 2012. PMID: 22548168 Free PMC article. - Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans.
Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Schon K, et al. J Neurosci. 2005 Oct 5;25(40):9112-23. doi: 10.1523/JNEUROSCI.1982-05.2005. J Neurosci. 2005. PMID: 16207870 Free PMC article. Clinical Trial.
References
- Awh E, Jonides J. Spatial working memory and spatial selective attention. In: Parasuraman R, editor. The attentive brain. MIT; Cambridge, MA: 1998. pp. 353–380.
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from positron tomography. Psychol Sci. 1996;7:25–31.
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. - PubMed
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp. 1995;3:93–106.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical