Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly - PubMed (original) (raw)
Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly
Z S Zhao et al. Mol Cell Biol. 2000 Sep.
Abstract
The p21-activated kinase PAK is targeted to focal complexes (FCs) through interactions with the SH3 domains of the PAK-interacting exchange factor PIX and Nck. PIX is a Rac GTP exchange factor that also binds the G-protein-coupled receptor kinase-interacting protein known as GIT1. Overexpression of GIT1 in fibroblasts or epithelial cells causes a loss of paxillin from FCs and stimulates cell motility. This is due to the direct interaction of a C-terminal 125-residue domain of GIT1 with paxillin, under the regulation of PIX. In its activated state, GIT1 can promote FC disassembly independent of actin-myosin contractile events. Additionally, GIT directly couples to a key component of FCs, focal adhesion kinase (FAK), via a conserved Spa2 homology domain. We propose that GIT1 and FAK cooperate to promote motility both by directly regulating focal complex dynamics and by the activation of Rac.
Figures
FIG. 1
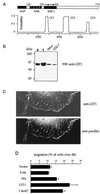
GIT1 expression stimulates cell motility. (A) Domain structure of GIT1. Regions with homology to recognized domains include the Arf GAP domain (GAP); a domain containing four ankyrin (ANK) repeats, and the direct repeat (arrows) comprising SHD-1. Three regions with coiled-coil potential include the conserved paxillin binding site at the C terminus. Residue numbers at the boundaries are marked. (B) GIT1 is present in cultured cells in FCs. Affinity-purified rabbit antibodies against GIT1646-770 identify a protein enriched in the testis, which is present as a single species in HeLa and COS-7 cells. B, brain; T, testis; WB, Western blot. (C) GIT1 localizes to FCs in COS-7 cells. Antipaxillin and anti-GIT1 identify punctate FCs in the center and at the periphery of the cells. (C) GIT1 is an efficient stimulator of cell motility. COS-7 cells were transfected with Flag-tagged expression constructs as shown and analyzed for migration through 8-μm membrane pores over 4 h under a soluble fibronectin-collagen gradient (see Materials and Methods).
FIG. 2
GIT1 promotes FC disassembly. (A) GIT1 expression causes the selective loss of paxillin from FCs which are faintly visible (arrows), while vinculin and phosphotyrosine (PY) levels are unaffected. The anti-Flag immunofluorescent photomicrographs of typical HeLa cells 4 h after injection are compared with images of uninjected controls. (B) The paxillin-binding domain GIT646-770 (left) does not affect antipaxillin staining. Coinjection of PIX and GIT1 (right) leads to focal adhesion loss, as assessed by antivinculin staining, and cell contraction. Injected cells are marked with stars. Bar, 10 μm. (C) Phase-contrast micrographs of cells showing induction of peripheral retraction. βPIX-expressing cells have small phase-dark ruffles, but coinjection of PIX plus GIT1 causes cell retraction (arrowhead). Membrane-tethered myrGIT1 similarly caused both cell body contraction and lamellipodia, driving neurite-like processes. Typical phenotypes were photographed 2 h after injection. (D) A C-terminal domain in GIT1 drives FC loss and cell contraction. The membrane-tethered constructs as shown were scored for cell contraction (B). The smallest myristoylated construct (646-770) was less efficient (++) in this regard. In each case, at least 15 microinjected cells were observed over a 3-h period.
FIG. 3
βPIX binds to GIT1 via the Spa2-related sequence. (A) The structural domains of βPIX include its N-terminal SH3 domain, which binds PAK, a catalytic Dbl homology (DH) region promoting nucleotide exchange on Rac1, and a C-terminal region (bar) which was used to bind GIT1 (B) by overlay. (B) GST fusion proteins corresponding to constructs 1 to 7 of GIT1 were separated on a 9% polyacrylamide gel and overlaid with PIX496-554 to localize the binding site. All positive binding constructs contain SHD-1 of GIT1254-376.
FIG. 4
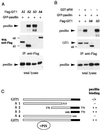
GIT1 association with paxillin LD4 is regulated by PIX binding. (A) Paxillin is immunoprecipitated with C-terminal constructs of GIT1. COS-7 cells were transfected with expression vectors containing Flag-tagged ΔGIT1 constructs depicted in panel C and GFP-paxillin. Western blots (WB) of anti-Flag immunoprecipitates or total lysates (50 μg per lane) were probed by antipaxillin. (The smallest Δ3 GIT1 [residues 646 to 770] is detected in separate gels but not shown here.) (B) Coexpression of PIX potentiates the ability of GIT1 to associate with paxillin. GIT1 or Δ4 and Δ3 represent cotransfection. A significant increase of paxillin (top) associated with Flag-GIT1 immunoprecipitates (IP) (middle) was observed when PIX was also present. Total GFP-paxillin expression is shown at the bottom. (C) Summary of constructs used and their ability to bind paxillin via the conserved C-terminal region (black).
FIG. 5
GIT1 directly binds FAK through SHD-1. (A) Flag-GIT1 or Flag-paxillin was coexpressed with GST-FAK, and the anti-Flag immunoprecipitates (IP) were analyzed for the presence of FAK. Little FAK was detected in paxillin immunoprecipitates compared to the amount in the total lysate. (B) Full-length FAK was expressed from pFlag-FAK vector. E. coli cell lysates (300 μl, 8 mg/ml) were passed through a 30-μl column of glutathione-Sepharose containing 2 mg of GST or GST/GIT11-376 per ml. rFak, recombinant FAK. (C) FAK and PIX can both associate with the GIT1 SHD-1. Flag-GIT1 constructs which were coexpressed with GST-FAK in COS-7 cells cover the entire GIT1 protein as shown schematically (domains as in Fig. 1). The Flag-tagged immunoprecipitates (lower panel) were used to detect the presence of FAK. The right panels show that Flag-PIX was able to immunoprecipitate FAK only in the presence of HA-GIT1. (D) FAK or Src was cotransfected with GIT1 and/or paxillin and analyzed for phosphotyrosine (PY) content. The amount of phosphotyrosine relative to anti-Flag signal indicates the efficiency with which GIT1 and paxillin are substrates for FAK (right lane).
FIG. 6
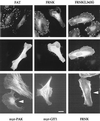
The FAK C-terminal domain can cause FC disassembly. (Top) FAK916-1053 and FRNK result in marked loss of FCs from HeLa cells as visualized by vinculin staining, but the mutant FRNK(L343S) is ineffective. Expression of each construct was by anti-Flag (middle). Typical cells are shown 2 h after injection with expression plasmid (100 μg/ml). FAK916-1053-expressing cells begin to narrow and undergo cell body retraction. Similar effects were noted with full-length FAK. (Bottom) Distribution of polymerized actin as visualized by phalloidin-fluorescein isothiocyanate in typical cells expressing myrαPAK, myrGIT1, or FRNK. Only in the first case is there loss of actin stress fibers. Arrowheads indicate injected cells. Bar, 10 μm.
FIG. 7
FAK and GIT1 can drive lamellipodial formation. Phase-contrast micrographs of HeLa cells microinjected with expression plasmids (0.1 μg/μl) as shown. FAK-expressing cells typically extend many phase-dark membrane ruffles (arrowheads), while myrGIT1 cells become bipolar with lamellipodia forming at the ends (arrows). Uninjected cells (U) do not show movement over this time frame. These new structures were completely blocked by coexpression of RacN17. The FRNK and myrGIT646-770 cells were captured at an intermediate stage of collapse: photomicrographs were taken with a 10-min interval approximately 1 h after injection.
FIG. 8
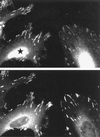
PAK can recruit GIT1 to FCs. (A) Increased FC-localized GIT1 in cells expressing the kinase inhibitor PAK83-149. The photomicrograph shows that in HeLa cells expressing the inhibitor (star), endogenous GIT1 staining is more concentrated in FCs relative to vinculin (B).
FIG. 9
PAK can recruit GIT1 to FCs. (A) Cdc42-induced peripheral FCs are stabilized by coexpression of membrane-targeted myrpaxillin. The typical rounded Cdc42G12V phenotype is seen 4 h after injection with or without coexpressed myrpaxillin. Note that with myrpaxillin the peripheral FCs are much denser. (B) COS-7 cells were transfected with Flag expression plasmids as shown. Motility of positively stained cells was assessed as for Fig. 1 (4 h after plating onto transwells). The top panel shows that myrpaxillin and the paxillin LD4 constructs effectively reduce Cdc42G12V-driven cell motility. KID, the PAK inhibitor αPAK83-149. (C) Smaller GIT1 and FAK constructs also promote motility. FAK1-427 corresponds to the band 4.1-like domain, and FAK916-1053 corresponds to the FAT domain.
FIG. 10
PAK and its putative roles in FC regulation. Events downstream of Cdc42 affect FCs. A cascade via PAK leads to paxillin dissociation from FCs, promoting their turnover. The PIX-GIT1 complex also activates Rac1, leading to formation of lamellipodia. PAK can down-regulate MLCK, which normally induces actin-myosin contractility and formation of FCs. ROK drives the process by inhibiting MLC dephosphorylation; MRCK also promotes FCs. FC turnover and resultant decrease in cell adherence, together with Rac1-driven peripheral changes, promote cell motility. FAK, which also act binds to GIT1, may act synergistically with the PAK/PIX/GIT1 pathway to facilitate motility. For details, see Discussion.
Similar articles
- Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway.
Brown MC, West KA, Turner CE. Brown MC, et al. Mol Biol Cell. 2002 May;13(5):1550-65. doi: 10.1091/mbc.02-02-0015. Mol Biol Cell. 2002. PMID: 12006652 Free PMC article. - p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase.
Lee J, Jung ID, Chang WK, Park CG, Cho DY, Shin EY, Seo DW, Kim YK, Lee HW, Han JW, Lee HY. Lee J, et al. Exp Cell Res. 2005 Jul 15;307(2):315-28. doi: 10.1016/j.yexcr.2005.03.028. Exp Cell Res. 2005. PMID: 15893751 - GIT1 activates p21-activated kinase through a mechanism independent of p21 binding.
Loo TH, Ng YW, Lim L, Manser E. Loo TH, et al. Mol Cell Biol. 2004 May;24(9):3849-59. doi: 10.1128/MCB.24.9.3849-3859.2004. Mol Cell Biol. 2004. PMID: 15082779 Free PMC article. - Paxillin interactions.
Turner CE. Turner CE. J Cell Sci. 2000 Dec;113 Pt 23:4139-40. doi: 10.1242/jcs.113.23.4139. J Cell Sci. 2000. PMID: 11069756 Review. - Pak to the future.
Bagrodia S, Cerione RA. Bagrodia S, et al. Trends Cell Biol. 1999 Sep;9(9):350-5. doi: 10.1016/s0962-8924(99)01618-9. Trends Cell Biol. 1999. PMID: 10461188 Review.
Cited by
- Identification of MYO18A as a novel interacting partner of the PAK2/betaPIX/GIT1 complex and its potential function in modulating epithelial cell migration.
Hsu RM, Tsai MH, Hsieh YJ, Lyu PC, Yu JS. Hsu RM, et al. Mol Biol Cell. 2010 Jan 15;21(2):287-301. doi: 10.1091/mbc.e09-03-0232. Epub 2009 Nov 18. Mol Biol Cell. 2010. PMID: 19923322 Free PMC article. - Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway.
Brown MC, West KA, Turner CE. Brown MC, et al. Mol Biol Cell. 2002 May;13(5):1550-65. doi: 10.1091/mbc.02-02-0015. Mol Biol Cell. 2002. PMID: 12006652 Free PMC article. - The PIX-GIT complex: a G protein signaling cassette in control of cell shape.
Frank SR, Hansen SH. Frank SR, et al. Semin Cell Dev Biol. 2008 Jun;19(3):234-44. doi: 10.1016/j.semcdb.2008.01.002. Epub 2008 Jan 20. Semin Cell Dev Biol. 2008. PMID: 18299239 Free PMC article. Review. - Regulation of actin cytoskeleton dynamics by Arf-family GTPases.
Myers KR, Casanova JE. Myers KR, et al. Trends Cell Biol. 2008 Apr;18(4):184-92. doi: 10.1016/j.tcb.2008.02.002. Epub 2008 Mar 6. Trends Cell Biol. 2008. PMID: 18328709 Free PMC article. Review. - Identification of two tyrosine residues required for the intramolecular mechanism implicated in GIT1 activation.
Totaro A, Astro V, Tonoli D, de Curtis I. Totaro A, et al. PLoS One. 2014 Apr 3;9(4):e93199. doi: 10.1371/journal.pone.0093199. eCollection 2014. PLoS One. 2014. PMID: 24699139 Free PMC article.
References
- Bagrodia S, Taylor S J, Jordon K A, Van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. - PubMed
- Bagrodia S, Bailey D, Lenard Z, Hart M, Guan J L, Premont R T, Taylor S J, Cerione R A. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J Biol Chem. 1999;274:22393–22400. - PubMed
- Bagrodia S, Cerione R A. Pak to the future. Trends Cell Biol. 1999;9:350–355. - PubMed
- Brown M C, Curtis M S, Turner C E. Paxillin LD motifs may define a new family of protein recognition domains. Nat Struct Biol. 1998;5:677–678. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous