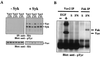Vav family proteins couple to diverse cell surface receptors - PubMed (original) (raw)
Vav family proteins couple to diverse cell surface receptors
S L Moores et al. Mol Cell Biol. 2000 Sep.
Abstract
Vav proteins are guanine nucleotide exchange factors for Rho family GTPases which activate pathways leading to actin cytoskeletal rearrangements and transcriptional alterations. Vav proteins contain several protein binding domains which can link cell surface receptors to downstream signaling proteins. Vav1 is expressed exclusively in hematopoietic cells and tyrosine phosphorylated in response to activation of multiple cell surface receptors. However, it is not known whether the recently identified isoforms Vav2 and Vav3, which are broadly expressed, can couple with similar classes of receptors, nor is it known whether all Vav isoforms possess identical functional activities. We expressed Vav1, Vav2, and Vav3 at equivalent levels to directly compare the responses of the Vav proteins to receptor activation. Although each Vav isoform was tyrosine phosphorylated upon activation of representative receptor tyrosine kinases, integrin, and lymphocyte antigen receptors, we found unique aspects of Vav protein coupling in each receptor pathway. Each Vav protein coprecipitated with activated epidermal growth factor and platelet-derived growth factor (PDGF) receptors, and multiple phosphorylated tyrosine residues on the PDGF receptor were able to mediate Vav2 tyrosine phosphorylation. Integrin-induced tyrosine phosphorylation of Vav proteins was not detected in nonhematopoietic cells unless the protein tyrosine kinase Syk was also expressed, suggesting that integrin activation of Vav proteins may be restricted to cell types that express particular tyrosine kinases. In addition, we found that Vav1, but not Vav2 or Vav3, can efficiently cooperate with T-cell receptor signaling to enhance NFAT-dependent transcription, while Vav1 and Vav3, but not Vav2, can enhance NFkappaB-dependent transcription. Thus, although each Vav isoform can respond to similar cell surface receptors, there are isoform-specific differences in their activation of downstream signaling pathways.
Figures
FIG. 1
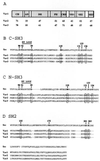
Sequence alignment of murine and human Vav3 proteins. Residues in human Vav3 (hVav3) identical to those in murine Vav3 (mVav3) are indicated with dashes. The boxes indicate structural domains: calponin homology (CH), acidic domain (AD), Dbl homology (DH), pleckstrin homology (PH), cysteine-rich domain (CRD), Src homology 3 (SH3), Src homology 2 (SH2).
FIG. 2
(A) Schematic features of Vav proteins and relative homology by domain. Percent identity with human Vav1 within each domain is displayed for human Vav2 and human Vav3. Domain abbreviations are as in Fig. 1. (B) Alignment of the SH3 domain of Src with the C-SH3 domains of Vav1, Vav2, and Vav3. The residues in Src predicted to contact a PXXP ligand are indicated, and the corresponding residues in the Vav proteins are boxed. (C) Alignment of the SH3 domain of Src with the N-SH3 domains of Vav1, Vav2, and Vav3. (D) Alignment of the SH2 domain of Src with the SH2 domains of Vav1, Vav2, and Vav3. The residues in Src predicted to contact the phosphotyrosine in the ligand are indicated, and the corresponding residues in the Vav proteins are boxed.
FIG. 3
EGF- and PDGF-induced tyrosine phosphorylation of Vav1, Vav2, and Vav3. Cos7 (A) or NIH 3T3 (B) cells were transfected with vector only, HA-Vav1, HA-Vav2, or HA-Vav3, serum starved, and then left untreated (−) or treated with growth factor (+). Vav proteins were immunoprecipitated (IP) with anti-HA antibody from cell lysates, and the level of phosphotyrosine was analyzed by immunoblotting with antiphosphotyrosine (anti-pTyr) antibody (upper panels). The relative amount of tyrosine-phosphorylated EGFR coprecipitated by each Vav protein varied from experiment to experiment. The mobilities of Vav proteins, EGFR, and PDGFR are indicated. Levels of Vav proteins were measured in duplicate anti-HA immunoprecipitations followed by immunoblotting with anti-HA antibody (lower panels). These data are representative of four independent experiments. (C) Cos7 cells were serum starved and then treated with 50 ng of EGF per ml for the indicated times (Timecourse) or treated with the indicated amounts of EGF for 2 min (Dose Response). Cells were then lysed, and proteins were immunoprecipitated with antibodies specific for Vav2. The level of phosphotyrosine was determined by immunoblotting with antiphosphotyrosine antibody. PI, preimmune serum. The mobilities of Vav2 and EGFR are indicated. The results are representative of two independent experiments. (D) Schematic diagram of the PDGFβR showing the tyrosine residues involved in this analysis. The tyrosine residue numbers as well as the subsequent three residues (+1, +2, and +3) are shown as the binding sites for PI 3′-kinase, RasGAP, SHP2, and PLCγ1 (24, 44). HepG2 cells expressing PDGFβR variants were serum starved and then left untreated (−) or treated with PDGF (+). Cells were then lysed, and proteins were immunoprecipitated with antibodies specific for Vav2 (or rabbit IgG for the control). The level of phosphotyrosine was determined by immunoblotting with antiphosphotyrosine antibody. Whole-cell extracts were immunoblotted with anti-PDGFβR antibodies to measure the level of each PDGFβR variant expressed. WT, wild type; F5, five tyrosines changed to phenylalanine. Derivative mutants have the following tyrosines added back to the F5 receptor: Y740/751, tyrosines 740 and 751; Y771, tyrosine 771; Y1009, tyrosine 1009; and Y1021, tyrosine 1021. The results shown are from a single representative experiment that was repeated three times with similar results.
FIG. 4
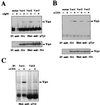
Expression of Syk allows integrin-induced tyrosine phosphorylation of Vav1, Vav2, and Vav3. (A) A5 CHO cells were transfected with plasmids expressing HA-Vav1, HA-Vav2, or HA-Vav3 alone (left) or in combination with pEMCV-Syk (right). Cells were serum starved and then placed in suspension (S) or plated on fibronectin (FN). Vav proteins were immunoprecipitated (IP) from cell lysates with anti-HA antibody, and the level of phosphotyrosine was analyzed by immunoblotting with antiphosphotyrosine (anti-pTyr) antibody. The mobilities of Vav proteins and Syk are indicated. Levels of Vav proteins in the immunoprecipitates were measured by reprobing the blots with anti-HA antibody. The results are representative of two independent experiments. (B) Cos7 cells were serum starved and then left untreated (−) or treated with EGF (+). Additional Cos7 cells were serum starved and then placed in suspension (S) or plated on fibronectin (FN). Cells were then lysed, and protein immunoprecipitated with antibodies specific for Vav2 (Vav2 IP) or focal adhesion kinase (Fak IP). The level of phosphotyrosine was determined by immunoblotting with antiphosphotyrosine antibody. The mobilities of Vav2 and Fak are indicated. These data are representative of three independent experiments.
FIG. 5
TCR- and BCR-induced tyrosine phosphorylation of Vav1, Vav2, and Vav3. Daudi B cells (A) or Jurkat T cells (B) were transfected with vector only, HA-Vav1, HA-Vav2, or HA-Vav3 and then left untreated (−) or treated with stimulatory antibodies (+). Vav proteins were immunoprecipitated (IP) from cell lysates with anti-HA antibody. The level of phosphotyrosine was analyzed by immunoblotting with antiphosphotyrosine (anti-pTyr) antibody (top), and the level of Vav proteins was measured by immunoblotting with anti-HA antibody (bottom). The mobilities of Vav proteins are indicated. The data are representative of three independent experiments. The higher level of Vav2 phosphorylation in the stimulated Daudi cells was not reproducible. (C) Jurkat T cells were left untreated (−) or treated with stimulatory antibodies (+), and endogenous Vav proteins were immunoprecipitated with antibodies specific for Vav1 or Vav2. The level of phosphotyrosine was analyzed by immunoblotting with antiphosphotyrosine antibody. The mobilities of Vav proteins are indicated.
FIG. 6
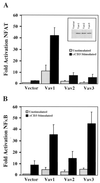
Potentiation of TCR-induced NFAT- and NFκB-dependent transcriptional activity by overexpression of Vav proteins. Jurkat T cells were cotransfected with an NFAT (A)- or NFκB- (B)-responsive luciferase reporter construct and either vector, HA-Vav1, HA-Vav2, or HA-Vav3. Cells were unstimulated or treated with stimulatory antibodies, as indicated, and lysed; then luciferase activity was measured. The results are shown as fold induction of luciferase activity compared with the activity in unstimulated cells cotransfected with vector and the luciferase reporter construct. Error bars represent standard errors of the means of three (A) or six (B) independent experiments. Inset in (A) shows the level of each Vav protein in cell lysates measured by immunoblotting with anti-HA antibody in a single representative experiment for assays in both panel A and panel B.
Similar articles
- Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins.
Liu BP, Burridge K. Liu BP, et al. Mol Cell Biol. 2000 Oct;20(19):7160-9. doi: 10.1128/MCB.20.19.7160-7169.2000. Mol Cell Biol. 2000. PMID: 10982832 Free PMC article. - Vav2 activates c-fos serum response element and CD69 expression but negatively regulates nuclear factor of activated T cells and interleukin-2 gene activation in T lymphocyte.
Tartare-Deckert S, Monthouel MN, Charvet C, Foucault I, Van Obberghen E, Bernard A, Altman A, Deckert M. Tartare-Deckert S, et al. J Biol Chem. 2001 Jun 15;276(24):20849-57. doi: 10.1074/jbc.M010588200. Epub 2001 Mar 21. J Biol Chem. 2001. PMID: 11262396 - Major transcript variants of VAV3, a new member of the VAV family of guanine nucleotide exchange factors.
Trenkle T, McClelland M, Adlkofer K, Welsh J. Trenkle T, et al. Gene. 2000 Mar 7;245(1):139-49. doi: 10.1016/s0378-1119(00)00026-3. Gene. 2000. PMID: 10713454 - Vav-family proteins in T-cell signalling.
Tybulewicz VL. Tybulewicz VL. Curr Opin Immunol. 2005 Jun;17(3):267-74. doi: 10.1016/j.coi.2005.04.003. Curr Opin Immunol. 2005. PMID: 15886116 Review. - Vav links antigen-receptor signaling to the actin cytoskeleton.
Fischer KD, Tedford K, Penninger JM. Fischer KD, et al. Semin Immunol. 1998 Aug;10(4):317-27. doi: 10.1006/smim.1998.0124. Semin Immunol. 1998. PMID: 9695188 Review.
Cited by
- Inflammatory breast cancer: relationship between growth factor signaling and motility in aggressive cancers.
van Golen KL. van Golen KL. Breast Cancer Res. 2003;5(3):174-9. doi: 10.1186/bcr598. Epub 2003 Apr 4. Breast Cancer Res. 2003. PMID: 12793901 Free PMC article. Review. - Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis.
Hunter SG, Zhuang G, Brantley-Sieders D, Swat W, Cowan CW, Chen J. Hunter SG, et al. Mol Cell Biol. 2006 Jul;26(13):4830-42. doi: 10.1128/MCB.02215-05. Mol Cell Biol. 2006. PMID: 16782872 Free PMC article. - Interaction between Rho GTPases and 14-3-3 Proteins.
Brandwein D, Wang Z. Brandwein D, et al. Int J Mol Sci. 2017 Oct 15;18(10):2148. doi: 10.3390/ijms18102148. Int J Mol Sci. 2017. PMID: 29036929 Free PMC article. Review. - Vav2 is required for cell spreading.
Marignani PA, Carpenter CL. Marignani PA, et al. J Cell Biol. 2001 Jul 9;154(1):177-86. doi: 10.1083/jcb.200103134. J Cell Biol. 2001. PMID: 11448999 Free PMC article. - Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis.
Albrecht B, Lairmore MD. Albrecht B, et al. Microbiol Mol Biol Rev. 2002 Sep;66(3):396-406, table of contents. doi: 10.1128/MMBR.66.3.396-406.2002. Microbiol Mol Biol Rev. 2002. PMID: 12208996 Free PMC article. Review.
References
- Abe K, Rossman K L, Liu B, Ritola K D, Chiang D, Campbell S L, Burridge K, Der C J. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–10149. - PubMed
- Adams J M, Houston H, Allen J, Lints T, Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992;7:611–618. - PubMed
- Bustelo X R, Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992;256:1196–1199. - PubMed
- Bustelo X R, Ledbetter J A, Barbacid M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992;356:68–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI20047/AI/NIAID NIH HHS/United States
- R01 AI020047/AI/NIAID NIH HHS/United States
- P01 HL59561/HL/NHLBI NIH HHS/United States
- R37 AI020047/AI/NIAID NIH HHS/United States
- R01 CA078773/CA/NCI NIH HHS/United States
- P01 HL059561/HL/NHLBI NIH HHS/United States
- CA78773/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous