In-depth mutational analysis of the promyelocytic leukemia zinc finger BTB/POZ domain reveals motifs and residues required for biological and transcriptional functions - PubMed (original) (raw)
In-depth mutational analysis of the promyelocytic leukemia zinc finger BTB/POZ domain reveals motifs and residues required for biological and transcriptional functions
A Melnick et al. Mol Cell Biol. 2000 Sep.
Abstract
The promyelocytic leukemia zinc finger (PLZF) protein is a transcription factor disrupted in patients with t(11;17)(q23;q21)-associated acute promyelocytic leukemia. PLZF contains an N-terminal BTB/POZ domain which is required for dimerization, transcriptional repression, formation of high-molecular-weight DNA-protein complexes, nuclear sublocalization, and growth suppression. X-ray crystallographic data show that the PLZF BTB/POZ domain forms an obligate homodimer via an extensive interface. In addition, the dimer possesses several highly conserved features, including a charged pocket, a hydrophobic monomer core, an exposed hydrophobic surface on the floor of the dimer, and two negatively charged surface patches. To determine the role of these structures, mutational analysis of the BTB/POZ domain was performed. We found that point mutations in conserved residues that disrupt the dimer interface or the monomer core result in a misfolded nonfunctional protein. Mutation of key residues from the exposed hydrophobic surface suggests that these are also important for the stability of PLZF complexes. The integrity of the charged-pocket region was crucial for proper folding of the BTB/POZ domain. In addition, the pocket was critical for the ability of the BTB/POZ domain to repress transcription. Alteration of charged-pocket residue arginine 49 to a glutamine (mutant R49Q) yields a domain that can still dimerize but activates rather than represses transcription. In the context of full-length PLZF, a properly folded BTB/POZ domain was required for all PLZF functions. However, PLZF with the single pocket mutation R49Q repressed transcription, while the double mutant D35N/R49Q could not, despite its ability to dimerize. These results indicate that PLZF requires the BTB/POZ domain for dimerization and the charged pocket for transcriptional repression.
Figures
FIG. 1
Structural features of the PLZF BTB/POZ dimer. (A) Ribbon view of the dimer, with one monomer in red and the other in blue. The location of the charged-pocket motif is identified by the black arrow, and the exposed hydrophobic surface is indicated by the green arrow. (B to D) Three views of the BTB/POZ surface colored by the electrostatic potential (−18 kT to +18 kT, where k is the Boltzman constant and T is the temperature in kelvins. Electropositive features are indicated in blue, electronegative features are indicated in red, and neutral surfaces are indicated in white. The asterisk denotes the negatively charged surface feature. (B) Side view, same orientation as in panel A. (C) View from below (as seen from the green arrow in panels A and B) showing the extended hydrophobic surface on the bottom of the dimer. (D) Top view (as seen from the black arrow in panels A and B), directly into the charged pocket. Both the charged pocket and the bottom hydrophobic surface are formed from residues contributed by both halves of the dimer. The figure was prepared using the graphics programs SETOR (14) and GRASP (36).
FIG. 2
Description and localization of BTB/POZ mutations. (A) List of the BTB/POZ mutations generated by PCR and their locations in the BTB/POZ dimer. Mutations which are located at the dimer interface are marked with an asterisk. (B) Ribbon view of the BTB/POZ dimer indicating the locations of residues selected for point mutations. The four surrounding diagrams denote, clockwise from top left, space-filling model of BTB/POZ dimer; BTB/POZΔ1–56 with deleted sequences shown in the space-filling mode; BTB/POZΔ83–114 with deleted sequences shown in the space-filling mode, and ALA48–52 with mutated sequences shown in the space-filling mode. (C) Sequence alignment of the PLZF BTB/POZ domain with other BTB/POZ domains showing conserved residues (asterisks) selected for mutational analysis. Darker shading indicates more highly conserved residues. m, mouse; ttk, tramtrack.
FIG. 3
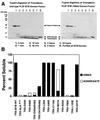
Biochemical analysis of BTB/POZ mutants. (A) Trypsin sensitivities of the wild-type (wt) PLZF BTB/POZ domain and mutant Y88A. Trypsin was added to thioredoxin fusion proteins of wild-type and mutant PLZF BTB/POZ domains at a ratio of 1:1,000 (wt/wt), and the digests were resolved by sodium dodecyl sulfate–14% polyacrylamide gel electrophoresis and staining with Coomassie blue. PLZF BTB/POZ domain mutant Y88A was degraded by trypsin, while the wild-type PLZF BTB/POZ domain remained intact for 24 h. Thioredoxin is resistant to trypsin under these conditions and serves as an internal control. (B and D) Solubility of thioredoxin (TRX)-BTB/POZ domain fusion proteins expressed in E. coli. Wild-type (WT) and mutant BTB/POZ fusion proteins were expressed to high levels in E. coli in all cases, but severe folding defects were identified in many of the mutants. Insoluble protein in the form of inclusion bodies was removed by centrifugation, and the soluble fractions of the fusion proteins in the supernatants were tested for their oligomeric state by gel filtration chromatography. Aggregates eluted in the voided volume of the column. As judged by molecular weight standards, the column could resolve monomers, dimers, and tetramers, although only dimers and aggregates larger than tetramers were observed. Note the strong correlation between aggregated protein and trypsin sensitivity, indicating that the soluble aggregates consisted of a misfolded BTB/POZ domain. (C) Thermal denaturation profiles of purified BTB/POZ domains, as measured by CD spectroscopy at 222 nm.
FIG. 3
Biochemical analysis of BTB/POZ mutants. (A) Trypsin sensitivities of the wild-type (wt) PLZF BTB/POZ domain and mutant Y88A. Trypsin was added to thioredoxin fusion proteins of wild-type and mutant PLZF BTB/POZ domains at a ratio of 1:1,000 (wt/wt), and the digests were resolved by sodium dodecyl sulfate–14% polyacrylamide gel electrophoresis and staining with Coomassie blue. PLZF BTB/POZ domain mutant Y88A was degraded by trypsin, while the wild-type PLZF BTB/POZ domain remained intact for 24 h. Thioredoxin is resistant to trypsin under these conditions and serves as an internal control. (B and D) Solubility of thioredoxin (TRX)-BTB/POZ domain fusion proteins expressed in E. coli. Wild-type (WT) and mutant BTB/POZ fusion proteins were expressed to high levels in E. coli in all cases, but severe folding defects were identified in many of the mutants. Insoluble protein in the form of inclusion bodies was removed by centrifugation, and the soluble fractions of the fusion proteins in the supernatants were tested for their oligomeric state by gel filtration chromatography. Aggregates eluted in the voided volume of the column. As judged by molecular weight standards, the column could resolve monomers, dimers, and tetramers, although only dimers and aggregates larger than tetramers were observed. Note the strong correlation between aggregated protein and trypsin sensitivity, indicating that the soluble aggregates consisted of a misfolded BTB/POZ domain. (C) Thermal denaturation profiles of purified BTB/POZ domains, as measured by CD spectroscopy at 222 nm.
FIG. 4
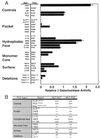
Yeast two-hybrid analysis of BTB/POZ mutant associations. (A) β-Galactosidase activity (± standard error of the mean [SEM]) of transformed yeast colonies depicted relative to the activity generated by wild-type BTB/POZ dimerization. Rows showing control experiments and pocket, hydrophobic-face, monomer core, surface, and deletion mutants are grouped separately. As positive controls, full-length GAL4 and wild-type PLZF and BTB/POZ (POZ) dimerization are shown. The wild-type BTB/POZ domain did not interact with simian virus 40 T antigen (SV40T) or p53, nor did it allow autoactivation of the yeast reporter genes. BTB/POZ mutant homodimerization and heterodimerization to wild-type BTB/POZ are shown. (B) Yeast two-hybrid analysis results tabulated according to the locations of the BTB/POZ mutations. Symbols refer to the relative strength of two-hybrid interaction.
FIG. 5
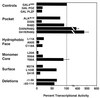
Transcriptional activities of BTB/POZ mutants. Mutant BTB/POZ domains were fused to the GAL4 DBD. These plasmids (400 ng) were transfected into 293T cells along with a reporter plasmid containing five GAL4 binding sites. A thymidine kinase-Renilla luciferase construct was also transfected as an internal control. The transcriptional effects are expressed as percent transcriptional activity (± SEM) compared to the activity of the GAL4 DBD alone (depicted as 100%).
FIG. 6
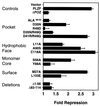
Transcriptional repression by full-length PLZF with BTB/POZ mutations. The mutant BTB/POZ domains were engineered into full-length PLZF, and 400 ng was transfected into 293T cells along with 100 ng of a reporter construct containing four PLZF binding sites. A thymidine kinase-Renilla luciferase construct (5 ng) was also transfected as an internal control. Results are expressed as fold repression compared to the repression obtained with the plasmid vector alone.
FIG. 7
HMW complex formation. PLZF proteins harboring wild-type and mutant BTB/POZ domains were expressed in 293T cells. Cell lysates were allowed to bind to an oligonucleotide containing PLZF binding sites from the IL-3 receptor α-chain promoter. The top arrow marks the shift in mobility caused by PLZF. The bottom arrow marks the mobility shift caused by PLZFΔBTB/POZ. The higher-mobility shift is seen in all PLZF-containing lanes, since there is low-level expression of a truncated protein when PLZF is transfected into cell cultures. Lane α PLZF SS, supershifting of PLZF with a monoclonal antibody.
FIG. 8
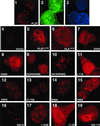
Immunolocalization of PLZF BTB/POZ mutants. Expression vectors for wild-type and mutant forms of PLZF were cotransfected along with GFP-spectrin in 293T cells. After incubation with PLZF antibody, a secondary Texas red-conjugated antibody was used to visualize PLZF. Cells were also stained with DAPI to precisely determine if proteins were confined to the nucleus. The slides were scanned at a magnification of ×1,000. Panels 1, 2, and 3, wild-type PLZF-transfected cells (1, PLZF-Texas red; 2, GFP; 3, DAPI); panels 4 to 19, mutant protein transfections (only Texas red panels are shown).
FIG. 9
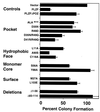
Suppression of colony formation. SaOS-2 cells were transfected with PLZF and the PLZF BTB/POZ mutants. Cells were then diluted and allowed to grow for 2 weeks in the presence of neomycin. The number of colonies (± SEM) was determined and compared to the number of colonies which formed from vector-transfected cells. This value was set at 100%, and the percent reduction of colony formation by each protein was plotted.
Similar articles
- Critical residues within the BTB domain of PLZF and Bcl-6 modulate interaction with corepressors.
Melnick A, Carlile G, Ahmad KF, Kiang CL, Corcoran C, Bardwell V, Prive GG, Licht JD. Melnick A, et al. Mol Cell Biol. 2002 Mar;22(6):1804-18. doi: 10.1128/MCB.22.6.1804-1818.2002. Mol Cell Biol. 2002. PMID: 11865059 Free PMC article. - Structure-function studies of the BTB/POZ transcriptional repression domain from the promyelocytic leukemia zinc finger oncoprotein.
Li X, Peng H, Schultz DC, Lopez-Guisa JM, Rauscher FJ 3rd, Marmorstein R. Li X, et al. Cancer Res. 1999 Oct 15;59(20):5275-82. Cancer Res. 1999. PMID: 10537309 - Overexpression, purification, characterization, and crystallization of the BTB/POZ domain from the PLZF oncoprotein.
Li X, Lopez-Guisa JM, Ninan N, Weiner EJ, Rauscher FJ 3rd, Marmorstein R. Li X, et al. J Biol Chem. 1997 Oct 24;272(43):27324-9. doi: 10.1074/jbc.272.43.27324. J Biol Chem. 1997. PMID: 9341182 - Nervous system regulated by POZ domain Krüppel-like zinc finger (POK) family transcription repressor RP58.
Okado H. Okado H. Br J Pharmacol. 2021 Feb;178(4):813-826. doi: 10.1111/bph.15265. Epub 2020 Dec 7. Br J Pharmacol. 2021. PMID: 32959890 Review. - Promyelocytic leukemia zinc finger controls type 2 immune responses in the lungs by regulating lineage commitment and the function of innate and adaptive immune cells.
Sha J, Zhang M, Feng J, Shi T, Li N, Jie Z. Sha J, et al. Int Immunopharmacol. 2024 Mar 30;130:111670. doi: 10.1016/j.intimp.2024.111670. Epub 2024 Feb 18. Int Immunopharmacol. 2024. PMID: 38373386 Review.
Cited by
- Genome-Wide Characterization of the BTB Gene Family in Poplar and Expression Analysis in Response to Hormones and Biotic/Abiotic Stresses.
Yue J, Dai X, Li Q, Wei M. Yue J, et al. Int J Mol Sci. 2024 Aug 21;25(16):9048. doi: 10.3390/ijms25169048. Int J Mol Sci. 2024. PMID: 39201733 Free PMC article. - Critical domains for NACC2-NTRK2 fusion protein activation.
Yang W, Meyer AN, Jiang Z, Jiang X, Donoghue DJ. Yang W, et al. PLoS One. 2024 Jun 27;19(6):e0301730. doi: 10.1371/journal.pone.0301730. eCollection 2024. PLoS One. 2024. PMID: 38935636 Free PMC article. - KCTD15 acts as an anti-tumor factor in colorectal cancer cells downstream of the demethylase FTO and the m6A reader YTHDF2.
Zhang FY, Wu L, Zhang TN, Chen HH. Zhang FY, et al. Commun Biol. 2024 Mar 4;7(1):262. doi: 10.1038/s42003-024-05880-9. Commun Biol. 2024. PMID: 38438714 Free PMC article. - Zinc Finger and BTB Domain-Containing 20: A Newly Emerging Player in Pathogenesis and Development of Human Cancers.
Liu J, Zhang H. Liu J, et al. Biomolecules. 2024 Feb 4;14(2):192. doi: 10.3390/biom14020192. Biomolecules. 2024. PMID: 38397429 Free PMC article. Review. - Loss-of-function variants in KCTD19 cause non-obstructive azoospermia in humans.
Liu J, Rahim F, Zhou J, Fan S, Jiang H, Yu C, Chen J, Xu J, Yang G, Shah W, Zubair M, Khan A, Li Y, Shah B, Zhao D, Iqbal F, Jiang X, Guo T, Xu P, Xu B, Wu L, Ma H, Zhang Y, Zhang H, Shi Q. Liu J, et al. iScience. 2023 Jun 28;26(7):107193. doi: 10.1016/j.isci.2023.107193. eCollection 2023 Jul 21. iScience. 2023. PMID: 37485353 Free PMC article.
References
- Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. 1995;6:1193–1198. - PubMed
- Aoki K, Meng G, Suzuki K, Takashi T, Kameoka Y, Nakahara K, Ishida R, Kasai M. RP58 associates with condensed chromatin and mediates a sequence-specific transcriptional repression. J Biol Chem. 1998;273:26698–26704. - PubMed
- Bardwell V J, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994;8:1664–1677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources