Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3 - PubMed (original) (raw)
Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3
J Li et al. EMBO J. 2000.
Abstract
We present evidence that both corepressors SMRT and N-CoR exist in large protein complexes with estimated sizes of 1.5-2 MDa in HeLa nuclear extracts. Using a combination of conventional and immunoaffinity chromatography, we have successfully isolated a SMRT complex and identified histone deacetylase 3 (HDAC3) and transducin (beta)-like I (TBL1), a WD-40 repeat-containing protein, as the subunits of the purified SMRT complex. We show that the HDAC3-containing SMRT and N-CoR complexes can bind to unliganded thyroid hormone receptors (TRs) in vitro. We demonstrate further that in Xenopus oocytes, both SMRT and N-CoR also associate with HDAC3 in large protein complexes and that injection of antibodies against HDAC3 or SMRT/N-CoR led to a partial relief of repression by unliganded TR/RXR. These findings thus establish both SMRT and N-CoR complexes as bona fide HDAC-containing complexes and shed new light on the molecular pathways by which N-CoR and SMRT function in transcriptional repression.
Figures
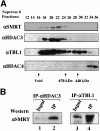
Fig. 1. SMRT and N-CoR proteins exist in large protein complexes with estimated sizes of 1.5–2 MDa. HeLa nuclear extract was fractionated with a Superose 6 gel filtration column. The indicated fractions were analyzed by western blotting using the antibodies indicated. The αN-CoR(C) antibody detected both SMRT and N-CoR proteins. The arrows at the bottom show the elution positions of calibration proteins of known molecular weights.
Fig. 2. Purification of a SMRT protein complex. (A) A diagram of the purification scheme for the SMRT complex. PC, phosphocellulose p11 resins; DEAE, DEAE–Sepharose Fast Flow resins. (B) Differential fractionation of SMRT and N-CoR proteins by a PC column. Note that αSMRT and αN-CoR(N) antibodies are specific for SMRT and N-CoR, respectively, whereas αN-CoR(C) antibody recognizes both SMRT and N-CoR. (C) A Coomassie blue-stained SDS–polyacrylamide gel showing the purified SMRT complex. The identity of the indicated subunits was determined by mass spectrometry and confirmed by western analyses as shown in (D). The Hsc70 protein was a contaminant because it also bound to the control IgG beads; 10% of the input (0.2 M DEAE fraction) was used in lane 1 in (D).
Fig. 3. Confirmation of the association of TBL1 and HDAC3 with SMRT complex. (A) HDAC3 and TBL1 co-fractionated with SMRT in gel filtration chromatography. HeLa nuclear extract was fractionated by using a Superose 6 gel filtration column as in Figure 1 and the indicated fractions were analyzed by western blotting using the antibodies indicated. (B) SMRT protein in HeLa nuclear extracts was co-immunoprecipitated by antibodies specific for HDAC3 and TBL1, respectively; 5% input HeLa nuclear extract was used in lanes 1 and 3.
Fig. 4. Both TBL1 and HDAC3 are also associated with the N-CoR protein complex(es). (A) N-CoR complex(es) was affinity-purified by both αN-CoR(N) and αN-CoR(C) antibody affinity columns and the associated proteins were analyzed by western blotting; 5% input HeLa nuclear extract was used in lanes 1 and 3. (B) The N-CoR protein in HeLa nuclear extracts was also co-immunoprecipitated by antibodies specific for HDAC3 and TBL1, respectively; 5% input HeLa nuclear extract was used in lanes 1 and 3.
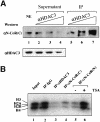
Fig. 5. The majority of the N-CoR and SMRT complexes are likely to contain HDAC3. (A) HeLa nuclear extracts were incubated with an increasing amount of HDAC3 antibody in an attempt to deplete HDAC3 from HeLa nuclear extracts. The levels of SMRT and N-CoR proteins in the supernatants or IP fractions were analyzed by western blotting using the αN-CoR(C) antibody, which detected both N-CoR and SMRT. The levels of HDAC3 in the supernatants are also shown, whereas the HDAC3 in IP fractions could not be determined by western analysis due to the overlapping of the signal from antibody heavy chain with that of HDAC3. (B) The immunopurified N-CoR complexes exhibited histone deacetylase activity. The N-CoR complex(es) or a mixture of SMRT and N-CoR complexes were pull-downed from HeLa nuclear extracts by using αN-CoR (N) and αN-CoR(C) affinity columns. The rabbit anti-rat IgG (lane 2) was used as negative control for IP, whereas αHDAC3 antibody served as a positive control. The purified calf thymus core histones were labeled with [3H]acetyl-CoA in vitro by using the purified recombinant p300 HAT domain and then used for measuring HDAC assay. In lane 6, TSA was added to a final concentration of 0.5 µM in the deacetylation assay.
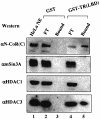
Fig. 6. HDAC3 may interact directly with N-CoR protein. (A) The schematic diagram illustrating the known functional domains of N-CoR protein and the five different N-CoR constructs. (B) In vitro translated N-CoR co-immunoprecipitated with the in vitro translated HDCA3. (C) The region between amino acids 1496 and 1965 of N-CoR interacted with MBP–HDAC3 in the in vitro pull-down assay. The indicated N-CoR fragments were translated and tested for binding to MBP–HDAC3; 5% input. (D) Yeast two-hybrid assay of the interactions between HDAC3 and different regions of the N-CoR protein. Yeast were transformed with the expression constructs for Gal4-HDAC3 and each of the N-CoR fragments fused with Gal4AD, and cell lysates were measured for activity of a β-galactosidase reporter.
Fig. 7. The HDAC3-containing SMRT and N-CoR complexes interact with unliganded TR. HeLa nuclear extracts were incubated with the immobilized GST control or GST–TR fusion protein. The super natants and bound fractions were then fractionated by SDS–PAGE and analyzed by western blotting using the antibodies indicated. The HeLa NE (nuclear extract) in lane 1 and FT (supernatant) in lanes 2 and 4 were equivalent to 20% of the HeLa nuclear extracts used for pull-down.
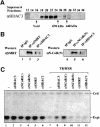
Fig. 8. The HDAC3-containing SMRT and N-CoR complexes are conserved during evolution and involved in the repression exerted by unliganded TR/RXR. (A) Gel filtration analysis of Xenopus oocyte extracts indicated that Xenopus HDAC3 also exists in large protein complexes with an estimated size of 1.5–2 MDa. (B) The association of Xenopus HDAC3 with Xenopus homologs of SMRT and N-CoR as revealed by co-immunoprecipitation experiments. Xenopus oocyte extracts were immunoprecipitated with a control rabbit anti-mouse IgG antibody (IgG), αSMRT, αN-CoR(N) and αHDAC3 antibodies as indicated, and the IP fractions were analyzed by western blotting. (C) Partial relief of the repression by unliganded TR/RXR through microinjection of antibodies against HDAC3 and SMRT/N-CoR. The reporter (pTRβA) was microinjected into Xenopus oocytes and the specific transcripts from this reporter were measured by primer extension analysis (Expt). The repression by unliganded TR/RXR was established by expression of TR and RXR in Xenopus oocytes by microinjection of their corresponding mRNAs. All antibodies used here were affinity-purified and injected at a concentration of 60 ng/µl (18.4 nl/oocyte). The control (Ctrl) was the primer extension product from the endogenous histone H4 mRNA using an H4 primer and served as a loading control.
Similar articles
- Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1.
Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Yoon HG, et al. EMBO J. 2003 Mar 17;22(6):1336-46. doi: 10.1093/emboj/cdg120. EMBO J. 2003. PMID: 12628926 Free PMC article. - Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development.
Tomita A, Buchholz DR, Shi YB. Tomita A, et al. Mol Cell Biol. 2004 Apr;24(8):3337-46. doi: 10.1128/MCB.24.8.3337-3346.2004. Mol Cell Biol. 2004. PMID: 15060155 Free PMC article. - Reading and function of a histone code involved in targeting corepressor complexes for repression.
Yoon HG, Choi Y, Cole PA, Wong J. Yoon HG, et al. Mol Cell Biol. 2005 Jan;25(1):324-35. doi: 10.1128/MCB.25.1.324-335.2005. Mol Cell Biol. 2005. PMID: 15601853 Free PMC article. - N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors.
Jones PL, Shi YB. Jones PL, et al. Curr Top Microbiol Immunol. 2003;274:237-68. doi: 10.1007/978-3-642-55747-7_9. Curr Top Microbiol Immunol. 2003. PMID: 12596910 Review. - Biological roles and mechanistic actions of co-repressor complexes.
Jepsen K, Rosenfeld MG. Jepsen K, et al. J Cell Sci. 2002 Feb 15;115(Pt 4):689-98. doi: 10.1242/jcs.115.4.689. J Cell Sci. 2002. PMID: 11865025 Review.
Cited by
- Molecular brake pad hypothesis: pulling off the brakes for emotional memory.
Vogel-Ciernia A, Wood MA. Vogel-Ciernia A, et al. Rev Neurosci. 2012;23(5-6):607-26. doi: 10.1515/revneuro-2012-0050. Rev Neurosci. 2012. PMID: 23096102 Free PMC article. Review. - General molecular biology and architecture of nuclear receptors.
Pawlak M, Lefebvre P, Staels B. Pawlak M, et al. Curr Top Med Chem. 2012;12(6):486-504. doi: 10.2174/156802612799436641. Curr Top Med Chem. 2012. PMID: 22242852 Free PMC article. Review. - 'Cut from the same cloth': Shared microsatellite variants among cancers link to ectodermal tissues-neural tube and crest cells.
Karunasena E, Mciver LJ, Bavarva JH, Wu X, Zhu H, Garner HR. Karunasena E, et al. Oncotarget. 2015 Sep 8;6(26):22038-47. doi: 10.18632/oncotarget.4194. Oncotarget. 2015. PMID: 26246470 Free PMC article. - The co-repressor SMRT delays DNA damage-induced caspase activation by repressing pro-apoptotic genes and modulating the dynamics of checkpoint kinase 2 activation.
Scafoglio C, Smolka M, Zhou H, Perissi V, Rosenfeld MG. Scafoglio C, et al. PLoS One. 2013 May 17;8(5):e59986. doi: 10.1371/journal.pone.0059986. Print 2013. PLoS One. 2013. PMID: 23690919 Free PMC article. - The MiDAC histone deacetylase complex is essential for embryonic development and has a unique multivalent structure.
Turnbull RE, Fairall L, Saleh A, Kelsall E, Morris KL, Ragan TJ, Savva CG, Chandru A, Millard CJ, Makarova OV, Smith CJ, Roseman AM, Fry AM, Cowley SM, Schwabe JWR. Turnbull RE, et al. Nat Commun. 2020 Jun 26;11(1):3252. doi: 10.1038/s41467-020-17078-8. Nat Commun. 2020. PMID: 32591534 Free PMC article.
References
- Alland L., Muhle,R., Hou,H.,Jr, Potes,J., Chin,L., Schreiber-Agus,N. and DePinho,R.A. (1997) Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature, 387, 49–55. - PubMed
- Baniahmad A., Steiner,C., Kohne,A.C. and Renkawitz,R. (1990) Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell, 61, 505–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials