A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion - PubMed (original) (raw)
A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion
D F Seals et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Yeast vacuoles undergo priming, docking, and homotypic fusion, although little has been known of the connections between these reactions. Vacuole-associated Vam2p and Vam6p (Vam2/6p) are components of a 65S complex containing SNARE proteins. Upon priming by Sec18p/NSF and ATP, Vam2/6p is released as a 38S subcomplex that binds Ypt7p to initiate docking. We now report that the 38S complex consists of both Vam2/6p and the class C Vps proteins [Reider, S. E. and Emr, S. D. (1997) Mol. Biol. Cell 8, 2307-2327]. This complex includes Vps33p, a member of the Sec1 family of proteins that bind t-SNAREs. We term this 38S complex HOPS, for homotypic fusion and vacuole protein sorting. This unexpected finding explains how Vam2/6p associates with SNAREs and provides a mechanistic link of the class C Vps proteins to Ypt/Rab action. HOPS initially associates with vacuole SNAREs in "cis" and, after release by priming, hops to Ypt7p, activating this Ypt/Rab switch to initiate docking.
Figures
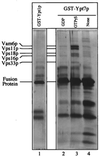
Figure 1
The class C Vps proteins associate with glutathione-immobilized GST-Ypt7p:GTP[γS]. Frozen BJ3505 vacuoles (300 μg) were diluted to 1.5 ml with PS buffer (see Materials and Methods) and sedimented (13,000 × g, 10 min, 4°C). Vacuole pellets were suspended in 500 μl of IP solubilization buffer [20 mM Hepes, pH 7.4/150 mM NaCl/10% glycerol/1% Triton X-100/1× protease inhibitor mixture (PIC; ref. 30)] and incubated on ice for 10 min. After sedimentation (13,000 × g, 10 min, 4°C), the solubilized vacuolar supernatant was transferred to fresh tubes containing GST-Ypt1p (lane 1) or GST-Ypt7p (lanes 2–4) that had been prebound to 40 μl (packed volume) of glutathione-Sepharose beads (Amersham Pharmacia) and preloaded with nucleotides (see below). [Fusion proteins underwent nucleotide exchange before incubation with the solubilized vacuolar supernatants. Each fusion protein (120 μg) was mixed by nutation with 40 μl of glutathione-Sepharose beads in 50 mM Tris·Cl, pH 8.0, for 20 min at room temperature. Bound material was sedimented (10,000 × g, 1 min, 4°C), and the supernatant was decanted. Beads were incubated (20 min, room temperature) in 500 μl of PS elution buffer (PS buffer with 2 M NaCl, 20 mM EDTA, 5 mM GDP, 1 mM DTT, and 1× PIC) and then sedimented and washed four times by resuspension in 1 ml of PS buffer and sedimentation (10,000 × g, 1 min, 4°C). Beads with fusion proteins were incubated (20 min, room temperature) with either 4 mM GTP[γS] (lanes 1 and 3), 4 mM GDP (lane 2), or no nucleotide (lane 4) in 500 μl of PS loading buffer (PS buffer containing 150 mM KCl, 4.5 mM MgCl2, 0.5 mM MnCl2, 1 mM DTT, and 1× PIC) and collected (10,000 × g, 1 min, 4°C).] Detergent extracts of vacuoles were mixed with beads by nutation for 2 h at 4°C. Unbound material was decanted from the beads after centrifugation (10,000 × g, 1 min, 4°C). Beads were resuspended twice in 1 ml of IP solubilization buffer and sedimented as above, and then bound proteins were eluted with 500 μl of PS elution buffer. Elution was by nutation for 15 min at room temperature followed by centrifugation. The eluate was precipitated by 12.5% trichloroacetic acid for 15 min on ice followed by centrifugation (13,000 × g, 15 min, 4°C). The pellet was resuspended in 1 ml of 80% acetone and sedimented as above. Protein pellets were dried and resuspended in 50 μl SDS/PAGE loading buffer, and polypeptides were separated on 10% acrylamide gels (35). Visualization was by silver staining (36); mass spectroscopy analysis (at the Howard Hughes Medical Institute/Keck Lab, Yale University) was performed on polypeptides extracted from a similar preparative gel stained with Coomassie brilliant blue.
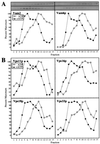
Figure 2
Cofractionation on sucrose velocity gradients of HOPS complex proteins. Freshly isolated BJ3505 vacuoles (400 μg) were diluted to 1.5 ml with PS buffer and sedimented (13,000 × g, 10 min, 4°C). Pellets were resuspended in 2 ml of either priming mixture [5 mM ATP, 5 mM MgCl2, 1 mg/ml creatine kinase, 100 mM creatine phosphate, 0.1× PIC, and 25 ng of his6-Sec18p/μg vacuolar protein (11)] or priming inhibition mixture (priming mixture, but with 0.16 units of apyrase/μg vacuolar protein substituting for ATP) and incubated for 10 min at 27°C. Vacuoles then were sedimented as above, resuspended in 1 ml of PS buffer, and sedimented. Vacuole pellets were suspended in 400 μl of PS solubilization buffer (PS buffer plus 1% Triton X-100 and 1× PIC) and incubated on ice for 10 min. After centrifugation (13,000 × g, 10 min, 4°C), the solubilized vacuolar supernatants were loaded on 11.6-ml continuous (40–10%, wt/vol) sucrose gradients in PS buffer plus 1% Triton X-100. Samples were centrifuged at 250,000 × g for 6 h at 4°C (SW 41 rotor; Beckman). Fractions of 800 μl were collected from the top of the gradient, and aliquots were separated by SDS/PAGE (10% acrylamide; ref. 35), analyzed by immunoblotting to the indicated proteins, and quantitated by scanning densitometry. ○, −ATP; ●, +ATP. (A) Vam2p and Vam6p. (B) Vps11p, Vps16p, Vps18p, and Vps33p.
Figure 3
Coimmunoprecipitation of HOPS complex subunits. (A) Detergent-solubilized vacuolar supernatants (500 μl) were prepared from 200 μg of frozen BJ3505 vacuoles as described in Fig. 1 (lane 1). Each vacuole extract was mixed by nutation for 2 h at 4°C with 20 μl (packed volume) of protein A-Sepharose CL-4B beads (Amersham Pharmacia), which had been cross-linked to affinity-purified antibodies to Vam2p (lane 2), Vam6p (lane 3), Vps11p (lane 4), Vps16p (lane 5), Vps18p (lane 6), Vps33p (lane 7), or IgG antibodies to E. coli SecE (lane 8). [Before mixing, beads were mock-eluted with 200 μl of 1% (wt/vol) SDS at 37°C for 10 min followed by sedimentation (10,000 × g, 1 min, 4°C). Beads were washed three times in 500 μl of IP solubilization buffer and then preblocked by a 15-min nutation in 500 μl of IP solubilization buffer containing 2% (wt/vol) BSA (Fraction V; Sigma). Beads were sedimented (10,000 × g, 1 min, 4°C) and then suspended twice in 500 μl of IP solubilization buffer and sedimented.] Beads were collected by sedimentation (10,000 × g, 1 min, 4°C) and washed two times by resuspension in 1 ml of IP solubilization buffer and sedimentation. Bound proteins were eluted with 200 μl of 1% (wt/vol) SDS as above. After sedimentation (10,000 × g, 1 min, 4°C) to remove beads, 180 μl of the eluate was supplemented with 45 μl of 5× SDS/PAGE loading buffer (45). Proteins were analyzed by SDS/PAGE (10% acrylamide) and immunoblotting. (B) Immunoprecipitations were as described in A, except that the antibodies were to Vps33p peptides (see Materials and Methods). After incubating the beads in IP solubilization buffer plus 2% (wt/vol) BSA and before mixing with vacuole detergent extracts, the beads were mixed with a 3:1 molar excess of either SecE peptide (ATV AFA REA ATE VRK VIW PTR QET C; SecE, lane 2) or Vps33 peptides (Vps33p, lane 3) for 1 h at room temperature.
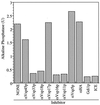
Figure 4
Antibody to each of the class C Vps proteins inhibits homotypic vacuole fusion. Homotypic vacuole fusion was assayed as described (30) but without cytosol. Before the preparation of the fusion assay mix, each vacuole population was incubated with 0.05 mg/ml affinity-purified antibodies either to the class C Vps proteins (Vps11p, Vps16p, Vps18p, and Vps33p), other Vps proteins (Vps5p, Vps17p, and Vps45p), or hemagglutinin (HA; epitope 12CA5). A separate reaction was treated with 60 μg/ml Gdi1p as a control for complete inhibition of fusion. One unit (U) of alkaline phosphatase activity produces 1 μmol of _p_-nitrophenol/min per μg BJ3505 vacuolar protein.
Similar articles
- The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein.
Price A, Seals D, Wickner W, Ungermann C. Price A, et al. J Cell Biol. 2000 Mar 20;148(6):1231-8. doi: 10.1083/jcb.148.6.1231. J Cell Biol. 2000. PMID: 10725336 Free PMC article. - New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion.
Wurmser AE, Sato TK, Emr SD. Wurmser AE, et al. J Cell Biol. 2000 Oct 30;151(3):551-62. doi: 10.1083/jcb.151.3.551. J Cell Biol. 2000. PMID: 11062257 Free PMC article. - A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking.
Ungermann C, Price A, Wickner W. Ungermann C, et al. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):8889-91. doi: 10.1073/pnas.160269997. Proc Natl Acad Sci U S A. 2000. PMID: 10908678 Free PMC article. - Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.
Wickner W. Wickner W. Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review. - Involvement of LMA1 and GATE-16 family members in intracellular membrane dynamics.
Elazar Z, Scherz-Shouval R, Shorer H. Elazar Z, et al. Biochim Biophys Acta. 2003 Aug 18;1641(2-3):145-56. doi: 10.1016/s0167-4889(03)00086-7. Biochim Biophys Acta. 2003. PMID: 12914955 Review.
Cited by
- Loss of the HOPS complex disrupts early-to-late endosome transition, impairs endosomal recycling and induces accumulation of amphisomes.
van der Beek J, de Heus C, Sanza P, Liv N, Klumperman J. van der Beek J, et al. Mol Biol Cell. 2024 Mar 1;35(3):ar40. doi: 10.1091/mbc.E23-08-0328. Epub 2024 Jan 10. Mol Biol Cell. 2024. PMID: 38198575 Free PMC article. - Relationship between the proteasomal system and autophagy.
Lilienbaum A. Lilienbaum A. Int J Biochem Mol Biol. 2013 Mar 31;4(1):1-26. Print 2013. Int J Biochem Mol Biol. 2013. PMID: 23638318 Free PMC article. - Identification of the ubiquitin ligase Triad1 as a regulator of endosomal transport.
Hassink G, Slotman J, Oorschot V, Van Der Reijden BA, Monteferrario D, Noordermeer SM, Van Kerkhof P, Klumperman J, Strous GJ. Hassink G, et al. Biol Open. 2012 Jun 15;1(6):607-14. doi: 10.1242/bio.2012778. Epub 2012 May 9. Biol Open. 2012. PMID: 23213454 Free PMC article. - Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex.
Lobingier BT, Merz AJ. Lobingier BT, et al. Mol Biol Cell. 2012 Dec;23(23):4611-22. doi: 10.1091/mbc.E12-05-0343. Epub 2012 Oct 10. Mol Biol Cell. 2012. PMID: 23051737 Free PMC article. - The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles.
Poupon V, Stewart A, Gray SR, Piper RC, Luzio JP. Poupon V, et al. Mol Biol Cell. 2003 Oct;14(10):4015-27. doi: 10.1091/mbc.e03-01-0040. Epub 2003 Jul 11. Mol Biol Cell. 2003. PMID: 14517315 Free PMC article.
References
- Rothman J H, Stevens T. Cell. 1986;47:1041–1051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases