Patterns of brain activation in people at risk for Alzheimer's disease - PubMed (original) (raw)
Comparative Study
Patterns of brain activation in people at risk for Alzheimer's disease
S Y Bookheimer et al. N Engl J Med. 2000.
Abstract
Background: The epsilon4 allele of the apolipoprotein E gene (APOE) is the chief known genetic risk factor for Alzheimer's disease, the most common cause of dementia late in life. To determine the relation between brain responses to tasks requiring memory and the genetic risk of Alzheimer's disease, we performed APOE genotyping and functional magnetic resonance imaging (MRI) of the brain in older persons with intact cognition.
Methods: We studied 30 subjects (age, 47 to 82 years) who were neurologically normal, of whom 16 were carriers of the APOE epsilon4 allele and 14 were homozygous for the APOE epsilon3 allele. The mean age and level of education were similar in the two groups. Patterns of brain activation during functional MRI scanning were determined while subjects memorized and recalled unrelated pairs of words and while subjects rested between such periods. Memory was reassessed in 14 subjects two years later.
Results: Both the magnitude and the extent of brain activation during memory-activation tasks in regions affected by Alzheimer's disease, including the left hippocampal, parietal, and prefrontal regions, were greater among the carriers of the APOE epsilon4 allele than among the carriers of the APOE epsilon3 allele. During periods of recall, the carriers of the APOE epsilon4 allele had a greater average increase in signal intensity in the hippocampal region (1.03 percent vs. 0.62 percent, P<0.001) and a greater mean (+/-SD) number of activated regions throughout the brain (15.9+/-6.2 vs. 9.4+/-5.5, P=0.005) than did carriers of the APOE epsilon3 allele. Longitudinal assessment after two years indicated that the degree of base-line brain activation correlated with degree of decline in memory.
Conclusions: Patterns of brain activation during tasks requiring memory differ depending on the genetic risk of Alzheimer's disease and may predict a subsequent decline in memory.
Figures
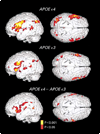
Figure 1. Statistical Parametric Maps of the Brain Used to Assess Subjects’ Performance on Memory-Activation Tests in Carriers of the APOE ε4 Allele and Carriers of the APOE ε3 Allele
Three-dimensional renditions of the surface of the brain are shown in gray, and colored areas indicate regions of significantly increased MRI signal intensity during learning or recall periods as compared with resting periods. The signal intensity increased significantly in the left inferior frontal region, the right prefrontal cortex, the transverse temporal gyri bilaterally, and the left posterior temporal and inferior parietal regions in both groups. However, both the extent and the intensity of activation were greater among the carriers of the APOE ε4 allele. The carriers of the APOE ε4 allele also had significant increases in the left parahippocampal region (Talairach and Tournoux atlas co-ordinates, −12, −38, and −10), the left dorsal prefrontal cortex (−56, 0, and 34; −50, −5, and 44), and in the inferior and superior parietal lobes (−48, −52, and 44 and −20, −80, and 26, respectively) and the anterior cingulate gyrus (12, 20, and 32). Direct comparisons of the carriers of the APOE ε4 allele and the carriers of the APOE ε3 allele (bottom panel, which shows the difference between the carriers) further demonstrated the greater extent and magnitude of activity in the left prefrontal region (atlas coordinates −60, 2, and 14 and −54, −18, and 32) and bilateral orbitofrontal, superior temporal, and inferior and superior parietal regions in the carriers of the APOE ε4 allele.
Figure 2. Examples of Activation Maps on Single MRI Planes
Two carriers of the APOE ε3 allele (Panel A) had fewer and less extensive areas of statistically significant activation (indicated in red) than did two carriers of the APOE ε4 allele (Panel B). The white lines indicate examples of regions of interest used for the analyses of data within subjects.
Figure 3. MRI Signal Intensity in the Hippocampus
Panel A shows the percent increases in signal intensity during learning or recall periods as compared with periods of rest, for the hippocampus and parahippocampal gyrus, averaged among subjects in each group. These increases are plotted for the nine minutes of the experiment; the peaks indicate periods of learning or recalling the word pairs, whereas the valleys indicate periods when the subjects were at rest. In both groups the signal intensity increased during the learning or recall periods as compared with the interspersed periods of rest, though these increases were larger in the carriers of the APOE ε4 allele. Panel B shows the mean (±SD) percent change in the MRI signal intensity in the hippocampus and parahippocampal gyrus during each of the six periods of recall. The response among the carriers of the APOE ε4 allele was consistently larger than the response among the carriers of the APOE ε3 allele.
Comment in
- Detection of preclinical Alzheimer's disease.
Skoog I. Skoog I. N Engl J Med. 2000 Aug 17;343(7):502-3. doi: 10.1056/NEJM200008173430709. N Engl J Med. 2000. PMID: 10944568 No abstract available.
Similar articles
- Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele.
Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mamourian AC, Belloni DR, Rhodes CH, McAllister TW. Wishart HA, et al. Am J Psychiatry. 2006 Sep;163(9):1603-10. doi: 10.1176/ajp.2006.163.9.1603. Am J Psychiatry. 2006. PMID: 16946187 - Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers.
Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Bäckman L, Nilsson LG, Petersson KM, Nyberg L. Lind J, et al. Brain. 2006 May;129(Pt 5):1240-8. doi: 10.1093/brain/awl054. Epub 2006 Mar 14. Brain. 2006. PMID: 16537568 - Brain-imaging surrogate markers for detection and prevention of age-related memory loss.
Small GW. Small GW. J Mol Neurosci. 2002 Aug-Oct;19(1-2):17-21. doi: 10.1007/s12031-002-0005-7. J Mol Neurosci. 2002. PMID: 12212776 Review. - Imaging studies and APOE genotype in persons at risk for Alzheimer's disease.
Scarmeas N, Stern Y. Scarmeas N, et al. Curr Psychiatry Rep. 2006 Feb;8(1):11-7. doi: 10.1007/s11920-006-0076-1. Curr Psychiatry Rep. 2006. PMID: 16513038 Free PMC article. Review.
Cited by
- Cerebral hyperactivation across the Alzheimer's disease pathological cascade.
Corriveau-Lecavalier N, Adams JN, Fischer L, Molloy EN, Maass A. Corriveau-Lecavalier N, et al. Brain Commun. 2024 Oct 25;6(6):fcae376. doi: 10.1093/braincomms/fcae376. eCollection 2024. Brain Commun. 2024. PMID: 39513091 Free PMC article. Review. - PS1/gamma-secretase acts as rogue chaperone of glutamate transporter EAAT2/GLT-1 in Alzheimer's disease.
Perrin F, Anderson LC, Mitchell SPC, Sinha P, Turchyna Y, Maesako M, Houser MCQ, Zhang C, Wagner SL, Tanzi RE, Berezovska O. Perrin F, et al. Acta Neuropathol Commun. 2024 Oct 21;12(1):166. doi: 10.1186/s40478-024-01876-y. Acta Neuropathol Commun. 2024. PMID: 39434170 Free PMC article. - Differences in Grey Matter Concentrations and Functional Connectivity between Young Carriers and Non-Carriers of the APOE ε4 Genotype.
Muñoz-Neira C, Zeng J, Kucikova L, Huang W, Xiong X, Muniz-Terrera G, Ritchie C, O'Brien JT, Su L. Muñoz-Neira C, et al. J Clin Med. 2024 Sep 3;13(17):5228. doi: 10.3390/jcm13175228. J Clin Med. 2024. PMID: 39274441 Free PMC article. - Early hippocampal hyperexcitability and synaptic reorganization in mouse models of amyloidosis.
Ray A, Loghinov I, Ravindranath V, Barth AL. Ray A, et al. iScience. 2024 Aug 2;27(9):110629. doi: 10.1016/j.isci.2024.110629. eCollection 2024 Sep 20. iScience. 2024. PMID: 39262788 Free PMC article. - Cerebral microvascular changes in healthy carriers of the APOE-ɛ4 Alzheimer's disease risk gene.
Aamand R, Rasmussen PM, Andersen KS, de Paoli S, Weitzberg E, Christiansen M, Lund TE, Østergaard L. Aamand R, et al. PNAS Nexus. 2024 Aug 23;3(9):pgae369. doi: 10.1093/pnasnexus/pgae369. eCollection 2024 Sep. PNAS Nexus. 2024. PMID: 39253395 Free PMC article.
References
- Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. - PubMed
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. - PubMed
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. - PubMed
- Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. - PubMed
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH052453-05/MH/NIMH NIH HHS/United States
- AG13308/AG/NIA NIH HHS/United States
- P01 NS026630/NS/NINDS NIH HHS/United States
- P50 AG005128/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- MH52453/MH/NIMH NIH HHS/United States
- P30 AG010123/AG/NIA NIH HHS/United States
- R01 NS031153/NS/NINDS NIH HHS/United States
- P60 AG011268-07/AG/NIA NIH HHS/United States
- R01 AG013308/AG/NIA NIH HHS/United States
- R01 MH052453/MH/NIMH NIH HHS/United States
- R01 AG013308-13/AG/NIA NIH HHS/United States
- P60 AG011268/AG/NIA NIH HHS/United States
- P50 AG005128-21/AG/NIA NIH HHS/United States
- P30 AG010123-03/AG/NIA NIH HHS/United States
- R01 NS031153-14/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous