Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells - PubMed (original) (raw)
Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells
V Brès et al. Br J Pharmacol. 2000 Aug.
Abstract
To characterize the volume-sensitive, osmolyte permeable anion channels responsible for the osmodependent release of taurine from supraoptic nucleus (SON) astrocytes, we investigated the pharmacological properties of the [(3)H]-taurine efflux from acutely isolated SON. Taurine release induced by hypotonic stimulus (250 mosmol l(-1)) was not antagonized by the taurine transporter blocker guanidinoethyl sulphonate, confirming the lack of implication of the transporter. The osmodependent release of taurine was blocked by a variety of Cl(-) channel inhibitors with the order of potency: NPPB>niflumic acid>DPC>DIDS>ATP. On the other hand, release of taurine was only weakly affected by other compounds (dideoxyforskolin, 4-bromophenacyl bromide, mibefradil) known to block volume-activated anion channels in other cell preparations, and was completely insensitive to tamoxifen, a broad inhibitor of these channels. Although the molecular identity of volume-sensitive anion channels is not firmly established, a few genes have been postulated as potential candidates to encode such channels. We checked the expression in the SON of three of them, ClC(3), phospholemman and VDAC(1), and found that the transcripts of these genes are found in SON neurons, but not in astrocytes. Similar observation was previously reported for ClC(2). In conclusion, the osmodependent taurine permeable channels of SON astrocytes display a particular pharmacological profile, suggesting the expression of a particular type or subtype of volume-sensitive anion channel, which is likely to be formed by yet unidentified proteins.
Figures
Figure 1
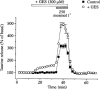
The osmodependent taurine efflux does not involve the taurine transporter. [3H]-taurine release from isolated SON in response to a 15% hypotonic stimulus. Application of the taurine transporter inhibitor GES increases both basal and evoked release, such that the ratio peak/basal stays constant (see text). Points are the mean of eight measurements. Standard errors are indicated when exceeding the size of the symbol.
Figure 2
Blockade of osmodependent taurine release by Cl− channel inhibitors. (a) Examples of blockade of both basal and hypotonicity-evoked release of [3H]-taurine by niflumic acid (_n_=4) and NPPB (_n_=6). (b) Inhibition curves of the evoked release for the compounds indicated. Solid lines are fits with a Hill equation (see Methods) that gave IC50 of 38±3 μ
M
(NPPB), 56±7 μ
M
(niflumic acid), 280±55 μ
M
(DPC), 869±22 μ
M
(DIDS) and 4.09±0.74 m
M
(ATP). Hill coefficients are 0.9–1.2. Each point is the mean of 4–8 measurements.
Figure 3
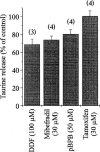
Weak sensitivity of taurine release to other anion channel blockers. Graph showing the peak of [3H]-taurine release evoked by a hypotonic stimulus in the presence of the compounds indicated. Release is expressed as per cent of control observed in the absence of the drug. Note the total lack of effect of tamoxifen. Number of observations is indicated above each bar.
Figure 4
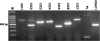
All ClCs, as well as phospholemman (PL) and VDAC1 genes are expressed in the SON. Electrophoresis gel showing the PCR products amplified from the nine mRNA encoding potential anion channels. Bands of expected size were amplified (see Table 1), and identity was checked by digestion with appropriate restriction enzymes. M, markers.
Figure 5
Neuronal localization of the expression of ClC3, phospholemman (PL) and VDAC1 in the SON. In situ hybridization showing the cellular expression of the three mRNA in the SON (a: CIC3; b: PL; c: VDAC1). Labelling is restricted to the large cell bodies located in the neuronal part of the nucleus. Note the absence of labelling in the ventral glia limitans where lie the somata of the astrocytes, as shown by the visualization of the expression of GFAP (d). OCh, optic chiasm.
Similar articles
- Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine.
Hussy N, Brès V, Rochette M, Duvoid A, Alonso G, Dayanithi G, Moos FC. Hussy N, et al. J Neurosci. 2001 Sep 15;21(18):7110-6. doi: 10.1523/JNEUROSCI.21-18-07110.2001. J Neurosci. 2001. PMID: 11549721 Free PMC article. - Swelling-activated efflux of taurine and other organic osmolytes in endothelial cells.
Manolopoulos VG, Voets T, Declercq PE, Droogmans G, Nilius B. Manolopoulos VG, et al. Am J Physiol. 1997 Jul;273(1 Pt 1):C214-22. doi: 10.1152/ajpcell.1997.273.1.C214. Am J Physiol. 1997. PMID: 9252459 - Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus.
Deleuze C, Duvoid A, Hussy N. Deleuze C, et al. J Physiol. 1998 Mar 1;507 ( Pt 2)(Pt 2):463-71. doi: 10.1111/j.1469-7793.1998.463bt.x. J Physiol. 1998. PMID: 9518705 Free PMC article. - Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure.
Hussy N, Deleuze C, Desarménien MG, Moos FC. Hussy N, et al. Prog Neurobiol. 2000 Oct;62(2):113-34. doi: 10.1016/s0301-0082(99)00071-4. Prog Neurobiol. 2000. PMID: 10828380 Review. - Intracellular signalling involved in volume regulatory decrease.
Hoffmann EK. Hoffmann EK. Cell Physiol Biochem. 2000;10(5-6):273-88. doi: 10.1159/000016356. Cell Physiol Biochem. 2000. PMID: 11125206 Review.
Cited by
- Nonsynaptic communication through ATP release from volume-activated anion channels in axons.
Fields RD, Ni Y. Fields RD, et al. Sci Signal. 2010 Oct 5;3(142):ra73. doi: 10.1126/scisignal.2001128. Sci Signal. 2010. PMID: 20923934 Free PMC article. - Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine.
Hussy N, Brès V, Rochette M, Duvoid A, Alonso G, Dayanithi G, Moos FC. Hussy N, et al. J Neurosci. 2001 Sep 15;21(18):7110-6. doi: 10.1523/JNEUROSCI.21-18-07110.2001. J Neurosci. 2001. PMID: 11549721 Free PMC article. - Functions of astrocytes and their potential as therapeutic targets.
Kimelberg HK, Nedergaard M. Kimelberg HK, et al. Neurotherapeutics. 2010 Oct;7(4):338-53. doi: 10.1016/j.nurt.2010.07.006. Neurotherapeutics. 2010. PMID: 20880499 Free PMC article. Review. - Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes.
Parkerson KA, Sontheimer H. Parkerson KA, et al. Glia. 2004 May;46(4):419-36. doi: 10.1002/glia.10361. Glia. 2004. PMID: 15095372 Free PMC article. - Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus.
Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Jia F, et al. J Neurosci. 2008 Jan 2;28(1):106-15. doi: 10.1523/JNEUROSCI.3996-07.2008. J Neurosci. 2008. PMID: 18171928 Free PMC article.
References
- BANDELARI U., ROY G. Anion channels for amino acids in MDCK cells. Am J. Physiol. 1992;263:C1200–C1207. - PubMed
- BOURQUE C.W., OLIET S.H.R. Osmoreceptors in the central nervous system. Annu. Rev. Physiol. 1997;59:601–619. - PubMed
- COLOMBINI M., BLACHY-DYSON E., FORTE M. VDAC, a channel in the outer mitochondrial membrane Ion channels 1996Plenum Publishing Corp.: New York, NY; 4, 169–202.ed. Narahashi, T. pp - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources