B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation - PubMed (original) (raw)
B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation
V Moulin et al. J Exp Med. 2000.
Abstract
Increasing evidence indicates that dendritic cells (DCs) are the antigen-presenting cells of the primary immune response. However, several reports suggest that B lymphocytes could be required for optimal T cell sensitization. We compared the immune responses of wild-type and B cell-deficient (muMT) mice, induced by antigen emulsified in adjuvant or pulsed on splenic dendritic cells. Our data show that lymph node cells from both control and muMT animals were primed, but each released distinct cytokine profiles. Lymph node T cells from control animals secreted interferon (IFN)-gamma, interleukin (IL)-2, and IL-4, whereas those from muMT mice produced IFN-gamma and IL-2 but no IL-4. To test whether B cells may influence the T helper cell type 1 (Th1)/Th2 balance by affecting the function of DCs, we immunized mice by transferring antigen-pulsed DCs from wild-type or mutant mice. Injection of control DCs induced the secretion of IL-4, IFN-gamma, and IL-2, whereas administration of DCs from muMT animals failed to sensitize cells to produce IL-4. Analysis of IL-12 production revealed that DCs from muMT mice produce higher levels of IL-12p70 than do DCs from wild-type animals. These data suggest that B lymphocytes regulate the capacity of DCs to promote IL-4 secretion, possibly by downregulating their secretion of IL-12, thereby favoring the induction of a nonpolarized immune response.
Figures
Figure 1
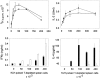
Responses of T cells in μMT and C57BL/6 mice to KLH. Wild-type (black symbols) or μMT (white symbols) mice were injected into the footpads with 100 μg KLH emulsified in CFA. Lymph nodes were harvested 5 d later and purified T cells (2 × 105/well) were incubated with graded numbers of irradiated, T-depleted spleen cells from C57BL/6 mice that had been pulsed with KLH (100 μg/ml). Proliferation and cytokine production were measured as indicated in Materials and Methods. Limits of detection: for IL-2, 0.16 U/ml; for IL-4, 31.25 pg/ml; for IFN-γ, 0.03 ng/ml. The data are representative of four independent experiments using pooled T cells from five C57BL/6 or μMT mice and shown as the mean of duplicate cultures ± SD.
Figure 2
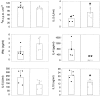
DCs from μMT mice do not prime for IL-4. C57BL/10J were injected with KLH-pulsed DCs from FLT3L-treated C57BL/10J (black symbols) or μMT (white symbols) mice into the hind and fore footpads. Lymph node cells were harvested 6 d later and cultured with KLH (5 μg/ml). Proliferative responses and cytokine secretion were measured as indicated in Materials and Methods. Each symbol represents the response of a single mouse and vertical bars are the mean results of five mice of each type (± SD). Five independent experiments were performed with similar results using DCs from untreated or FLT3L-treated mice. Limits of detection: for IL-2, 0.16 U/ml; IL-4 and IL-5, 62.5 pg/ml; IFN-γ, 0.3 ng/ml; IL-10, 1 ng/ml. The groups indicated by single and double asterisks are significantly different from control (Student's t test, P < 0.05 and 0.01, respectively; n = 5).
Figure 3
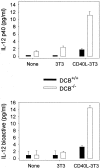
Production of IL-12 by DCs. DCs purified from untreated WT (DCB+/+) or μMT (DCB−/−) mice were cultured with medium alone (None) or with fibroblasts transfected (CD40L-3T3) or not (3T3) with CD40L. 48-h and 72-h supernatants were tested for IL-12 p40 and p70 content, respectively. Limits of detection: for p40, 0.3 pg/ml; p70, 0.13 pg/ml. Data shown are the mean ± SD of duplicate cultures. Three independent experiments were performed with similar results, using DCs purified from untreated or FLT3L-treated mice.
Figure 4
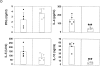
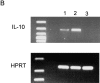
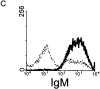
Role of IL-10. (A) RT-PCR analysis of IL-10 expression was performed in spleen cells from untreated wild-type (lanes 1 and 2) and μMT (lanes 3 and 4) mice, using IL-10 and HPRT primers. Anti-CD3–injected mice were used as positive control (lane 5). Left and right sides represent the amplification by PCR of 3λ and 1λ cDNA, respectively. Similar data were obtained in three independent experiments. (B) RT-PCR analysis of IL-10 expression was performed in unseparated spleen cells (lane 1) and splenic CD19+ cell population (lane 2), which contains 98% B lymphocytes as assessed by FACS™ analysis for IgM expression (C). The T cell hybridoma 3B4.15 was used as negative control (panel B, lane 3). (D) DCs were purified from C57BL/10 (black symbols) or IL-10 knockout (white symbols) mice that were treated for 9 d consecutively with FLT3L. KLH-pulsed DCs were injected into the hind and fore footpads of wild-type recipient animals. Lymph nodes were harvested 6 d later and cultured with KLH (5 μg/ml). The supernatants were collected and cytokine activities were determined by ELISA. Detection limits: for IFN-γ, 0.3 ng/ml; IL-4, 31.3 pg/ml; IL-5, 31.3 U/ml; IL-10, 1 ng/ml. Each symbol represents production of cytokine of individual animals, and vertical bars are the mean results of five mice. Two independent experiments were performed with similar results. The groups indicated by the double asterisks are significantly different from control (Student's t test, P < 0.01, n = 5).
Figure 4
Role of IL-10. (A) RT-PCR analysis of IL-10 expression was performed in spleen cells from untreated wild-type (lanes 1 and 2) and μMT (lanes 3 and 4) mice, using IL-10 and HPRT primers. Anti-CD3–injected mice were used as positive control (lane 5). Left and right sides represent the amplification by PCR of 3λ and 1λ cDNA, respectively. Similar data were obtained in three independent experiments. (B) RT-PCR analysis of IL-10 expression was performed in unseparated spleen cells (lane 1) and splenic CD19+ cell population (lane 2), which contains 98% B lymphocytes as assessed by FACS™ analysis for IgM expression (C). The T cell hybridoma 3B4.15 was used as negative control (panel B, lane 3). (D) DCs were purified from C57BL/10 (black symbols) or IL-10 knockout (white symbols) mice that were treated for 9 d consecutively with FLT3L. KLH-pulsed DCs were injected into the hind and fore footpads of wild-type recipient animals. Lymph nodes were harvested 6 d later and cultured with KLH (5 μg/ml). The supernatants were collected and cytokine activities were determined by ELISA. Detection limits: for IFN-γ, 0.3 ng/ml; IL-4, 31.3 pg/ml; IL-5, 31.3 U/ml; IL-10, 1 ng/ml. Each symbol represents production of cytokine of individual animals, and vertical bars are the mean results of five mice. Two independent experiments were performed with similar results. The groups indicated by the double asterisks are significantly different from control (Student's t test, P < 0.01, n = 5).
Figure 4
Role of IL-10. (A) RT-PCR analysis of IL-10 expression was performed in spleen cells from untreated wild-type (lanes 1 and 2) and μMT (lanes 3 and 4) mice, using IL-10 and HPRT primers. Anti-CD3–injected mice were used as positive control (lane 5). Left and right sides represent the amplification by PCR of 3λ and 1λ cDNA, respectively. Similar data were obtained in three independent experiments. (B) RT-PCR analysis of IL-10 expression was performed in unseparated spleen cells (lane 1) and splenic CD19+ cell population (lane 2), which contains 98% B lymphocytes as assessed by FACS™ analysis for IgM expression (C). The T cell hybridoma 3B4.15 was used as negative control (panel B, lane 3). (D) DCs were purified from C57BL/10 (black symbols) or IL-10 knockout (white symbols) mice that were treated for 9 d consecutively with FLT3L. KLH-pulsed DCs were injected into the hind and fore footpads of wild-type recipient animals. Lymph nodes were harvested 6 d later and cultured with KLH (5 μg/ml). The supernatants were collected and cytokine activities were determined by ELISA. Detection limits: for IFN-γ, 0.3 ng/ml; IL-4, 31.3 pg/ml; IL-5, 31.3 U/ml; IL-10, 1 ng/ml. Each symbol represents production of cytokine of individual animals, and vertical bars are the mean results of five mice. Two independent experiments were performed with similar results. The groups indicated by the double asterisks are significantly different from control (Student's t test, P < 0.01, n = 5).
Figure 4
Role of IL-10. (A) RT-PCR analysis of IL-10 expression was performed in spleen cells from untreated wild-type (lanes 1 and 2) and μMT (lanes 3 and 4) mice, using IL-10 and HPRT primers. Anti-CD3–injected mice were used as positive control (lane 5). Left and right sides represent the amplification by PCR of 3λ and 1λ cDNA, respectively. Similar data were obtained in three independent experiments. (B) RT-PCR analysis of IL-10 expression was performed in unseparated spleen cells (lane 1) and splenic CD19+ cell population (lane 2), which contains 98% B lymphocytes as assessed by FACS™ analysis for IgM expression (C). The T cell hybridoma 3B4.15 was used as negative control (panel B, lane 3). (D) DCs were purified from C57BL/10 (black symbols) or IL-10 knockout (white symbols) mice that were treated for 9 d consecutively with FLT3L. KLH-pulsed DCs were injected into the hind and fore footpads of wild-type recipient animals. Lymph nodes were harvested 6 d later and cultured with KLH (5 μg/ml). The supernatants were collected and cytokine activities were determined by ELISA. Detection limits: for IFN-γ, 0.3 ng/ml; IL-4, 31.3 pg/ml; IL-5, 31.3 U/ml; IL-10, 1 ng/ml. Each symbol represents production of cytokine of individual animals, and vertical bars are the mean results of five mice. Two independent experiments were performed with similar results. The groups indicated by the double asterisks are significantly different from control (Student's t test, P < 0.01, n = 5).
Figure 5
B cells from recipient animals are required for IL-4 priming. KLH-pulsed DCs from untreated C57BL/10 mice were injected into the hind and fore footpads of wild-type (black symbols) or μMT (white symbols) recipients. Lymph nodes were harvested 6 d later and purified T cells were cultured with various numbers of γ-irradiated, T cell–depleted spleen cells from C57BL/10 mice, which have been pulsed with KLH. Proliferation and lymphokine production were measured as indicated in Materials and Methods. Detection limits: for IL-2, 0.16 U/ml; IFN-γ, 0.3 ng/ml; IL-4, 16 pg/ml. The results represent the mean of duplicate cultures (± SD). Similar data were obtained in three independent experiments.
Similar articles
- Splenic dendritic cells pulsed with Ixodes ricinus tick saliva prime naive CD4+T to induce Th2 cell differentiation in vitro and in vivo.
Mejri N, Brossard M. Mejri N, et al. Int Immunol. 2007 Apr;19(4):535-43. doi: 10.1093/intimm/dxm019. Epub 2007 Mar 6. Int Immunol. 2007. PMID: 17344202 - Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy.
Feili-Hariri M, Falkner DH, Morel PA. Feili-Hariri M, et al. J Leukoc Biol. 2005 Sep;78(3):656-64. doi: 10.1189/jlb.1104631. Epub 2005 Jun 16. J Leukoc Biol. 2005. PMID: 15961574 - Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells.
Iwasaki A, Kelsall BL. Iwasaki A, et al. J Exp Med. 1999 Jul 19;190(2):229-39. doi: 10.1084/jem.190.2.229. J Exp Med. 1999. PMID: 10432286 Free PMC article. - Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans.
Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Lyakh L, et al. Immunol Rev. 2008 Dec;226:112-31. doi: 10.1111/j.1600-065X.2008.00700.x. Immunol Rev. 2008. PMID: 19161420 Free PMC article. Review. - The role of B cells in the programming of T cells for IL-4 synthesis.
Mason D. Mason D. J Exp Med. 1996 Mar 1;183(3):717-9. doi: 10.1084/jem.183.3.717. J Exp Med. 1996. PMID: 8642274 Free PMC article. Review. No abstract available.
Cited by
- Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances.
Zhao L, Jin S, Wang S, Zhang Z, Wang X, Chen Z, Wang X, Huang S, Zhang D, Wu H. Zhao L, et al. Signal Transduct Target Ther. 2024 Aug 28;9(1):225. doi: 10.1038/s41392-024-01947-5. Signal Transduct Target Ther. 2024. PMID: 39198425 Free PMC article. Review. - High dimensional proteomic mapping of bone marrow immune characteristics in immune thrombocytopenia.
Liu FQ, Qu QY, Lei Y, Chen Q, Chen YX, Li ML, Sun XY, Wu YJ, Huang QS, Fu HX, Kong Y, Li YY, Wang QF, Huang XJ, Zhang XH. Liu FQ, et al. Sci China Life Sci. 2024 Aug;67(8):1635-1647. doi: 10.1007/s11427-023-2520-4. Epub 2024 Apr 19. Sci China Life Sci. 2024. PMID: 38644444 - The Roles of Various Immune Cell Populations in Immune Response against Helminths.
Lekki-Jóźwiak J, Bąska P. Lekki-Jóźwiak J, et al. Int J Mol Sci. 2023 Dec 28;25(1):420. doi: 10.3390/ijms25010420. Int J Mol Sci. 2023. PMID: 38203591 Free PMC article. Review. - Exploring the dual role of B cells in solid tumors: implications for head and neck squamous cell carcinoma.
Bao J, Betzler AC, Hess J, Brunner C. Bao J, et al. Front Immunol. 2023 Oct 5;14:1233085. doi: 10.3389/fimmu.2023.1233085. eCollection 2023. Front Immunol. 2023. PMID: 37868967 Free PMC article. Review. - Therapeutic strategies for gastric cancer targeting immune cells: Future directions.
Zhao Y, Bai Y, Shen M, Li Y. Zhao Y, et al. Front Immunol. 2022 Sep 23;13:992762. doi: 10.3389/fimmu.2022.992762. eCollection 2022. Front Immunol. 2022. PMID: 36225938 Free PMC article. Review.
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
- Kalinski P., Hilkens C.M.U., Wierenga E.A., Kapsenberg M.L. T-cell priming by type-1 and type-2 polarized dendritic cellsthe concept of a third signal. Immunol. Today. 1999;20:561–567. - PubMed
- De Smedt T., Van Mechelen M., De Becker G., Urbain J., Leo O., Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 1997;27:1229–1235. - PubMed
- Ron Y., Sprent J. T cell priming in vivoa major role for B cells in presenting antigen to T cells in lymph nodes. J. Immunol. 1987;138:2848–2856. - PubMed
- Kurt-Jones E.A., Liano D., HayGlass K.A., Benacerraf B., Sy M.S., Abbas A.K. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J. Immunol. 1988;140:3773–3778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical