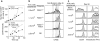Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells - PubMed (original) (raw)
Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells
B K Cho et al. J Exp Med. 2000.
Abstract
The developmental requirements for immunological memory, a central feature of adaptive immune responses, is largely obscure. We show that as naive CD8 T cells undergo homeostasis-driven proliferation in lymphopenic mice in the absence of overt antigenic stimulation, they progressively acquire phenotypic and functional characteristics of antigen-induced memory CD8 T cells. Thus, the homeostasis-induced memory CD8 T cells express typical memory cell markers, lyse target cells directly in vitro and in vivo, respond to lower doses of antigen than naive cells, and secrete interferon gamma faster upon restimulation. Like antigen-induced memory T cell differentiation, the homeostasis-driven process requires T cell proliferation and, initially, the presence of appropriate restricting major histocompatibility complexes, but it differs by occurring without effector cell formation and without requiring interleukin 2 or costimulation via CD28. These findings define repetitive cell division plus T cell receptor ligation as the basic requirements for naive to memory T cell differentiation.
Figures
Figure 1
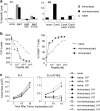
Naive CD8 T cells spontaneously differentiate into memory cells after transfer into RAG-1−/− recipients. Total lymph node cells or CD8+CD44− cells from 2C/RAG or B6 mice were adoptively transferred into syngeneic RAG-1−/− recipients. After 3 wk or more, CD8 T cells from these nonimmunized recipients were analyzed for memory cell phenotype and function (non-immunized). For comparison, antigen-induced memory cells were generated by immunizing some recipients of 2C cells with SIYRYYGL peptide in CFA and studying them at least 4 mo later (immunized). Naive 2C and B6 T cells were from 2C/RAG and normal B6 donors, respectively. (a) Persisting CD8 T cells in nonimmunized recipients acquire a cell surface phenotype characteristic of memory T cells. Lymph node cells from RAG-1−/− recipients or from naive donors were analyzed by flow cytometry using antibodies to TCR and CD8 plus antibodies to CD44, Ly-6C, IL-2Rβ, or LFA-1. The expression of CD44, Ly-6C, IL-2Rβ, and LFA-1 on TCR+CD8+ T cells is shown as histograms. (b) CD8 T cells from nonimmunized recipients are rapidly induced to express IFN-γ. Intracellular (IC) IFN-γ expression by CD8+ T cells is shown as histograms: bold outline, with anti-CD3ε stimulation (8 h); filled, without stimulation.
Figure 2
Functional comparison of naive and memory 2C T cells. Naive and memory 2C cells from either immunized or nonimmunized recipients were generated as in the legend to Fig. 1. (a) CD8 T cells from nonimmunized recipients are directly cytolytic. Purified 2C T cells from RAG-1−/− recipients or naive donors were added at an E/T ratio of 2.5:1 in the presence of 1 nM SIYRYYGL peptide (pept). Target cell lysis depended on the presence of the peptide and was blocked by antibody (1B2) to the 2C TCR (30 μg/ml) or by EGTA (4 mM). The cytolytic activity of purified CD8+ cells from B6 recipients and donors was assayed by ConA-mediated killing of target cells at an E/T ratio of 1:1. Target cell lysis required the presence of ConA (5 μg/ml) and was inhibited by α-methylmannoside (αmm, 5%) or EGTA. (b) CD8 T cells from nonimmunized recipients downmodulate TCR and upregulate CD69 in response to lower concentrations of antigenic peptide than naive cells. Lymph node cells from naive 2C/RAG mice and immunized and nonimmunized RAG-1−/− recipients of 2C cells were incubated with irradiated B6 splenocytes in the presence of different concentrations of SIYRYYGL peptide for 4 h. Cells were analyzed for the levels of 2C TCR, CD69, and CD8. Fluorescence intensity (geometric mean) of TCR staining of CD8+ cells and percentages of TCR+CD8+ T cells that are CD69+ are shown as a function of peptide concentrations. (c) CD8 T cells from nonimmunized recipients are more effective than naive cells in tumor rejection. B6 mice were implanted with 3 × 106 EL4 tumor cells on one side and 3 × 106 EL4 tumor cells expressing the SIYRYYGL epitope (EL4-SYRGL) on the other side. 2 d later, mice were either not transferred with any 2C cells or were transferred with indicated numbers of naive 2C cells, memory 2C cells from immunized mice, or memory 2C cells from nonimmunized mice. Tumor sizes (in cm2) generated from transferred EL4 or EL4-SYRGL tumor cells are shown at different days after implantation. When 104 and 105 T cells were transferred, memory 2C cells from immunized recipients were significantly more effective than those from nonimmunized recipients in rejecting EL4-SYRGL tumor at day 23 (not shown).
Figure 3
Naive CD8 T cells undergo homeostasis-mediated proliferation in RAG-1−/− recipients. (a) Transferred T cells undergo proliferation in RAG-1−/− recipients in the absence of apparent antigen stimulation. The number of surviving T cells (TCR+CD8+) in the lymph nodes and spleen and the intensity of CD44 expression (geometric mean) on these T cells are shown as a function of time. Lymph node (naive) refers to 2C T cells from naive donor mice. (b) Proliferation is mediated by homeostatic mechanisms. Different numbers of CFSE-labeled naive 2C T cells were adoptively transferred into syngeneic normal B6 recipients, RAG-1−/− recipients, or RAG-1−/− recipients that had received naive 2C cells and peptide immunization 4 mo before the transfer of CFSE-labeled cells (“filled”). The intensity of CFSE on 2C cells from different recipients is shown. (c) Memory T cell differentiation is linked to the extent of cell division. The levels of CD44 and intracellular IFN-γ expression after anti-CD3ε stimulation (8 h) are shown on CD8+ T cells. Naive (control) indicates B6 donor cells.
Figure 4
Transferred T cells differentiate progressively into memory cells without becoming activated effector cells. (a) Transferred T cells progressively acquire the capacity for rapid induction of IFN-γ expression. Lymph node cells from 2C or B6 donors were labeled with CFSE and transferred into RAG-1−/− recipients for 9 d. Then, lymph node cells from the recipients were cultured in the presence or absence of anti-CD3ε antibody for 8 h and assayed for intracellular IFN-γ expression. The expression of IFN-γ by CD8+ T cells is shown as a function of CFSE intensity (left). The percentage of IFN-γ+ cells within each cell division cohort (middle) is shown as a function of the number of cell divisions (right). IFN-γ+ cells are those above the horizontal line (left). Similar to CD8 cells, CD4 T cells from B6 lymph node also proliferated in the RAG-1−/− recipients and acquired high levels of CD44 and rapid IFN-γ expression after anti-CD3ε stimulation (data not shown). (b and c) Progressive differentiation of memory cell phenotype occurs in the absence of activated effector cell formation. (b) CD44 expression versus CFSE intensity is shown for CD8+ cells by two-dimensional dot plots (left). The percentage of CD44+ T cells is also shown as a function of the number of cell divisions (right). CD44+ cells are those above the horizontal line (left). (c) CD25 expression versus CFSE intensity is shown for CD8+ cells. (d and e) Transferred T cells differentiate into activated effector cells after immunization. 2 d after the transfer of CFSE-labeled 2C T cells, RAG-1−/− recipients were immunized with SIYRYYGL peptide in complete Freund's adjuvant. 3 d later, 2C cells from lymph nodes of the immunized recipients were assayed directly for IFN-γ, CD25, and CD44. (d) The expression of IFN-γ by CD8+ 2C T cells from immunized recipients (effector) is compared with that of naive 2C T cells. (e) The expression of IFN-γ, CD25, and CD44 by CD8+ effector T cells is shown as a function of CFSE intensity.
Figure 5
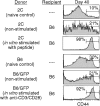
Proliferation is required for memory CD8 T cell differentiation. Lymph node cells from 2C donors or B6/GFP donors were either directly transferred into syngeneic B6 recipients (non-stimulated), or were activated in vitro with an antigenic peptide (10 nM SIYRYYGL for 2C cells) or with anti-CD3ε plus anti-CD28 (10 μg/ml each for B6/GFP), allowed to proliferate for 3 d, and then transferred into normal B6 recipients. 40 d after transfer, CD8 T cells from lymph nodes were analyzed for CD44 expression, gating on CD8+2C+ (for 2C) and CD8+GFP+ (for B6 T cells) cells.
Figure 6
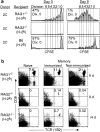
Requirements for homeostasis-driven proliferation. (a) Homeostasis-driven proliferation requires the presence of both “space” and the correct MHC. Proliferation of CD8+TCR+ cells in various recipients is shown as histograms of CFSE profiles. RAG-2−/− (H-2k) recipients were treated with anti-Ly49G2 (200 μg intraperitoneally) to deplete NK cells 1 d before the transfer and another 100 μg on the day of transfer. H-2k was not recognized by 2C cells as shown by mixed lymphocyte reaction (data not shown). (b) Comparison of survival of naive and memory 2C T cells in recipients with “incorrect” MHCs. An equal number of naive and memory 2C T cells (1 × 106) was transferred into either syngeneic (H-2b) RAG-1−/− recipients or RAG-2−/− recipients having an incorrect MHC class I (H-2k). 9 and 14 d after the transfer, splenocytes and lymph node cells were assayed for CD8 and TCR (1B2) expression by flow cytometry. Numbers indicate the percentages of CD8+TCR+ cells in lymph node. A similar result was also obtained in the spleen (not shown). The memory 2C T cells were generated as described in Materials and Methods and RAG-2−/− (H-2k) recipients were treated with anti-Ly49G2 antibody as above.
Comment in
- Homeostatic T cell proliferation: how far can T cells be activated to self-ligands?
Surh CD, Sprent J. Surh CD, et al. J Exp Med. 2000 Aug 21;192(4):F9-F14. doi: 10.1084/jem.192.4.f9. J Exp Med. 2000. PMID: 10952731 Free PMC article. Review. No abstract available.
Similar articles
- Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation.
Goldrath AW, Bogatzki LY, Bevan MJ. Goldrath AW, et al. J Exp Med. 2000 Aug 21;192(4):557-64. doi: 10.1084/jem.192.4.557. J Exp Med. 2000. PMID: 10952725 Free PMC article. - IFN-gamma receptor signaling regulates memory CD8+ T cell differentiation.
Sercan O, Stoycheva D, Hämmerling GJ, Arnold B, Schüler T. Sercan O, et al. J Immunol. 2010 Mar 15;184(6):2855-62. doi: 10.4049/jimmunol.0902708. Epub 2010 Feb 17. J Immunol. 2010. PMID: 20164422 - Spontaneous Proliferation of CD4+ T Cells in RAG-Deficient Hosts Promotes Antigen-Independent but IL-2-Dependent Strong Proliferative Response of Naïve CD8+ T Cells.
Kim J, Lee JY, Cho K, Hong SW, Kim KS, Sprent J, Im SH, Surh CD, Cho JH. Kim J, et al. Front Immunol. 2018 Aug 23;9:1907. doi: 10.3389/fimmu.2018.01907. eCollection 2018. Front Immunol. 2018. PMID: 30190718 Free PMC article. - Memory phenotype of CD8+ T cells in MHC class Ia-deficient mice.
Kurepa Z, Su J, Forman J. Kurepa Z, et al. J Immunol. 2003 Jun 1;170(11):5414-20. doi: 10.4049/jimmunol.170.11.5414. J Immunol. 2003. PMID: 12759416 - Multiple effects of immunostimulatory DNA on T cells and the role of type I interferons.
Sun S, Zhang X, Tough D, Sprent J. Sun S, et al. Springer Semin Immunopathol. 2000;22(1-2):77-84. doi: 10.1007/s002810000028. Springer Semin Immunopathol. 2000. PMID: 10944802 Review.
Cited by
- An in vivo IL-7 requirement for peripheral Foxp3+ regulatory T cell homeostasis.
Kim GY, Ligons DL, Hong C, Luckey MA, Keller HR, Tai X, Lucas PJ, Gress RE, Park JH. Kim GY, et al. J Immunol. 2012 Jun 15;188(12):5859-66. doi: 10.4049/jimmunol.1102328. Epub 2012 May 16. J Immunol. 2012. PMID: 22593613 Free PMC article. - IL-15, in synergy with RAE-1ɛ, stimulates TCR-independent proliferation and activation of CD8(+) T cells.
Qian L, Zhang Y, Pan XY, Ji MC, Gong WJ, Tian F. Qian L, et al. Oncol Lett. 2012 Feb;3(2):472-476. doi: 10.3892/ol.2011.495. Epub 2011 Nov 25. Oncol Lett. 2012. PMID: 22740934 Free PMC article. - Transgene-derived overexpression of miR-17-92 in CD8+ T-cells confers enhanced cytotoxic activity.
Kosaka A, Ohkuri T, Ikeura M, Kohanbash G, Okada H. Kosaka A, et al. Biochem Biophys Res Commun. 2015 Mar 13;458(3):549-554. doi: 10.1016/j.bbrc.2015.02.003. Epub 2015 Feb 10. Biochem Biophys Res Commun. 2015. PMID: 25677619 Free PMC article. - γ-Herpesvirus reactivation differentially stimulates epitope-specific CD8 T cell responses.
Freeman ML, Burkum CE, Jensen MK, Woodland DL, Blackman MA. Freeman ML, et al. J Immunol. 2012 Apr 15;188(8):3812-9. doi: 10.4049/jimmunol.1102787. Epub 2012 Mar 9. J Immunol. 2012. PMID: 22407914 Free PMC article. - A deficiency in nucleoside salvage impairs murine lymphocyte development, homeostasis, and survival.
Choi O, Heathcote DA, Ho KK, Müller PJ, Ghani H, Lam EW, Ashton-Rickardt PG, Rutschmann S. Choi O, et al. J Immunol. 2012 Apr 15;188(8):3920-7. doi: 10.4049/jimmunol.1102587. Epub 2012 Mar 9. J Immunol. 2012. PMID: 22407915 Free PMC article.
References
- Goldrath A.W., Bevan M.J. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–261. - PubMed
- Tanchot C., Lemonnier F.A., Pérarnau B., Freitas A.A., Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. - PubMed
- Viret C., Wong F.S., Janeway C.A., Jr. Designing and maintaining the mature TCR repertoirethe continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. - PubMed
- Ernst B., Lee D.-S., Chang J.M., Sprent J., Surh C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials