Binding site of brefeldin A at the interface between the small G protein ADP-ribosylation factor 1 (ARF1) and the nucleotide-exchange factor Sec7 domain - PubMed (original) (raw)
Binding site of brefeldin A at the interface between the small G protein ADP-ribosylation factor 1 (ARF1) and the nucleotide-exchange factor Sec7 domain
S Robineau et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Sec7 domains (Sec7d) catalyze the exchange of guanine nucleotide on ARFs. Recent studies indicated that brefeldin A (BFA) inhibits Sec7d-catalyzed nucleotide exchange on ARF1 in an uncompetitive manner by trapping an early intermediate of the reaction: a complex between GDP-bound ARF1 and Sec7d. Using (3)H-labeled BFA, we show that BFA binds to neither isolated Sec7d nor isolated ARF1-GDP, but binds to the transitory Sec7d-ARF1-GDP complex and stabilizes it. Two pairs of residues at positions 190-191 and 198-208 (Arno numbering) in Sec7d contribute equally to the stability of BFA binding, which is also sensitive to mutation of H80 in ARF1. The catalytic glutamic (E156) residue of Sec7d is not necessary for BFA binding. In contrast, BFA does not bind to the intermediate catalytic complex between nucleotide-free ARF1 and Sec7d. These results suggest that, on initial docking steps between ARF1-GDP and Sec7d, BFA inserts like a wedge between the switch II region of ARF1-GDP and a surface encompassing residues 190-208, at the border of the characteristic hydrophobic groove of Sec7d. Bound BFA would prevent the switch regions of ARF1-GDP from reorganizing and forming tighter contacts with Sec7d and thereby would maintain the bound GDP of ARF1 at a distance from the catalytic glutamic finger of Sec7d.
Figures
Figure 1
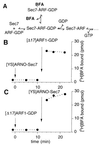
BFA binds to a complex between Sec7d and [Δ17]ARF1. (A) Proposed scheme for the inhibition of Sec7d by BFA as suggested by kinetic studies of Sec7d-catalyzed nucleotide exchange on ARF1 (15, 23). (B and C) Direct measurements of [3H]BFA binding. The sample (100 μl) contained initially 25 μM [3H]BFA (253 dpm/pmol) in buffer and was incubated at 27°C. At the indicated times, [YS]Arno-Sec7 (5 μM) and [Δ17]ARF1–GDP (10 μM) were added sequentially from concentrated stock solutions to the sample. Note the different order of protein additions between B and C. To determine the binding of [3H]BFA, 15-μl aliquots were withdrawn from the sample and filtered immediately onto cellulose filters, and the amount of [3H]BFA trapped on the filter was measured (see Materials and Methods). The amount of protein in each 15-μl aliquot was 75 pmol of [YS]Arno-Sec7 and/or 150 pmol of [Δ17]ARF1.
Figure 2
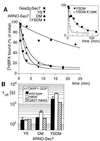
Effect of point mutations in Sec7d and [Δ17]ARF1–GDP on the dissociation rate of BFA from the abortive [Δ17]ARF1–GDP–Sec7d complex. (A) Time course of BFA dissociation. In a preliminary step, Sec7d (5 μM) was incubated for 10 min at 27°C with 10 μM [Δ17]ARF1–GDP/20 μM GDP/25 μM [3H]BFA. At zero time, 300 μM unlabeled BFA was added and the kinetics of [3H]BFA dissociation was followed by measuring the bound radioactivity in 15-μl aliquots. The Sec7d used were Gea2p-Sec7 (■); [YS]Arno-Sec7 (▾); [DM]Arno-Sec7 (▴); and [YSDM]Arno-Sec7 (●). (Inset) Time course of BFA dissociation from the abortive complex with [YSDM]Arno-Sec7 (●) on a different time scale as well as the dissociation of BFA from the abortive complex with [YSDM-E156K]Arno-Sec7 (○). (B) Conditional effect of the H80A mutation of [Δ17]ARF1–GDP on the dissociation rate of BFA. The time course of [3H]BFA dissociation from various abortive [Δ17]ARF1–GDP–Sec7d complexes carrying the indicated mutations in [Δ17]ARF1 and in Arno-Sec7 was measured as in A. The time constant, τoff(1/e), for BFA dissociation was determined by fitting the experimental points with a single exponential. Error bars show the standard error for two to four independent experiments. Note that the H80A mutation of [Δ17]ARF1–GDP slowed down the dissociation of BFA only when Sec7d bore the DM mutation.
Figure 3
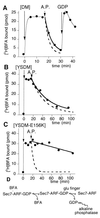
BFA does not bind to the nucleotide-free complex between [Δ17]ARF1 and Sec7d. The sample (200 μl) contained initially 10 μM [Δ17]ARF1–GDP and 25 μM [3H]BFA. The binding of 25 μM [3H]BFA was initiated at zero time by the addition of Arno-Sec7 (5 μM) carrying either the DM mutations (A), the YSDM mutations (B), or the YSDM + E156K mutations (C). At t = 17 min, alkaline phosphatase (200 units/ml) was added to the sample. In A, 2 mM GDP was further added at t = 31 min. Each experimental point corresponded to the bound radioactivity in a 15-μl aliquot. The continuous thick lines show an exponential fit of the data points after the addition of alkaline phosphatase. The dashed lines report the time course of [3H]BFA dissociation as measured by exchange with unlabeled BFA and measured in Fig. 2. Note the good correlation between the two fits (A and B) except for the complex with [YSDM-E156K]Arno-Sec7 (C). These experiments suggest that once BFA has bound to a Sec7d–ARF1–GDP complex, the release of GDP and its hydrolysis by alkaline phosphatase requires first the dissociation of BFA from the complex and then the destabilization of GDP by the glutamic finger of Sec7d (D).
Figure 4
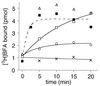
Mutation of the glutamic finger of Sec7d increases the rate of BFA binding. Association kinetics of [3H]BFA (15 μM) with 0.5 μM [YSDM]Arno-Sec7 (white symbols) or [YSDN-E156K]Arno-Sec7 (■), in the presence of 0 (×), 1 (○), 2 (■, □), or 5 μM (▵) [Δ17]ARF1–GDP. The reaction was initiated by the addition of Sec7d. The t = 0 point corresponds to the initial level of bound [3H]BFA just before the addition of Sec7d and represents the nonspecific binding of BFA. Bound radioactivity was determined on 15-μl aliquots (7.5 pmol of Sec7d).
Figure 5
Model for the binding of BFA to the Sec7d–ARF1–GDP complex. A docking approach of free ARF1–GDP (26) onto the Sec7d of Arno (3), based on previously identified contacts (dotted lines; ref. 6) is shown. Note the complementarity of shape between the interacting surfaces: the protuberant switch regions of ARF1–GDP and the groove of Sec7d. This might represent the first step of the nucleotide-exchange reaction, i.e., when ARF1–GDP contacts Sec7d. In a second step, a dramatic conformational change occurs in ARF1, which is coupled to the penetration of the glutamic finger (E156) of Sec7d into the nucleotide-binding site of ARF1 and the destabilization of GDP (7). BFA inhibits this second step by binding specifically to the Sec7d–ARF1–GDP complex. The positions of residues in ARF1 and Sec7d that are important for the binding of BFA are shown in green. This includes H80 in helix α2 of ARF1 and residues F/
Y
190, A/
S
191, S/
D
198, and P/
M
208 in helix αH of Sec7d (underlined amino acids favored the binding of BFA). In the nucleotide-free Sec7d–ARF1 complex, helix α2 of ARF1 interacts extensively with helix αH of Sec7d. In contrast, when ARF1–GDP contacts Sec7d, helix α2 of ARF1 would be at a distance from helix αH of Sec7d, and BFA could insert between the two helices like a wedge. This insertion would block the subsequent reorganization of the ARF1/Sec7d interface.
Similar articles
- Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism.
Mossessova E, Corpina RA, Goldberg J. Mossessova E, et al. Mol Cell. 2003 Dec;12(6):1403-11. doi: 10.1016/s1097-2765(03)00475-1. Mol Cell. 2003. PMID: 14690595 - Kinetics of interaction between ADP-ribosylation factor-1 (Arf1) and the Sec7 domain of Arno guanine nucleotide exchange factor, modulation by allosteric factors, and the uncompetitive inhibitor brefeldin A.
Rouhana J, Padilla A, Estaran S, Bakari S, Delbecq S, Boublik Y, Chopineau J, Pugnière M, Chavanieu A. Rouhana J, et al. J Biol Chem. 2013 Feb 15;288(7):4659-72. doi: 10.1074/jbc.M112.391748. Epub 2012 Dec 19. J Biol Chem. 2013. PMID: 23255605 Free PMC article. - Integrating three views of Arf1 activation dynamics.
Robert CH, Cherfils J, Mouawad L, Perahia D. Robert CH, et al. J Mol Biol. 2004 Apr 2;337(4):969-83. doi: 10.1016/j.jmb.2004.01.052. J Mol Biol. 2004. PMID: 15033364 - Arf, Sec7 and Brefeldin A: a model towards the therapeutic inhibition of guanine nucleotide-exchange factors.
Zeghouf M, Guibert B, Zeeh JC, Cherfils J. Zeghouf M, et al. Biochem Soc Trans. 2005 Dec;33(Pt 6):1265-8. doi: 10.1042/BST0331265. Biochem Soc Trans. 2005. PMID: 16246094 Review. - Activation of toxin ADP-ribosyltransferases by eukaryotic ADP-ribosylation factors.
Moss J, Vaughan M. Moss J, et al. Mol Cell Biochem. 1999 Mar;193(1-2):153-7. Mol Cell Biochem. 1999. PMID: 10331652 Review.
Cited by
- Golgi traffic and integrity depend on N-myristoyl transferase-1 in Arabidopsis.
Renna L, Stefano G, Majeran W, Micalella C, Meinnel T, Giglione C, Brandizzi F. Renna L, et al. Plant Cell. 2013 May;25(5):1756-73. doi: 10.1105/tpc.113.111393. Epub 2013 May 14. Plant Cell. 2013. PMID: 23673980 Free PMC article. - Protein-protein interfaces in molecular glue-induced ternary complexes: classification, characterization, and prediction.
Rui H, Ashton KS, Min J, Wang C, Potts PR. Rui H, et al. RSC Chem Biol. 2023 Jan 3;4(3):192-215. doi: 10.1039/d2cb00207h. eCollection 2023 Mar 8. RSC Chem Biol. 2023. PMID: 36908699 Free PMC article. Review. - Coordinated Activation of ARF1 GTPases by ARF-GEF GNOM Dimers Is Essential for Vesicle Trafficking in Arabidopsis.
Brumm S, Singh MK, Nielsen ME, Richter S, Beckmann H, Stierhof YD, Fischer AM, Kumaran M, Sundaresan V, Jürgens G. Brumm S, et al. Plant Cell. 2020 Aug;32(8):2491-2507. doi: 10.1105/tpc.20.00240. Epub 2020 Jun 2. Plant Cell. 2020. PMID: 32487565 Free PMC article. - Endogenous mutant Huntingtin alters the corticogenesis via lowering Golgi recruiting ARF1 in cortical organoid.
Liu Y, Chen X, Ma Y, Song C, Ma J, Chen C, Su J, Ma L, Saiyin H. Liu Y, et al. Mol Psychiatry. 2024 Oct;29(10):3024-3039. doi: 10.1038/s41380-024-02562-0. Epub 2024 Apr 23. Mol Psychiatry. 2024. PMID: 38654124 Free PMC article. - GBF1 and Arf1 interact with Miro and regulate mitochondrial positioning within cells.
Walch L, Pellier E, Leng W, Lakisic G, Gautreau A, Contremoulins V, Verbavatz JM, Jackson CL. Walch L, et al. Sci Rep. 2018 Nov 20;8(1):17121. doi: 10.1038/s41598-018-35190-0. Sci Rep. 2018. PMID: 30459446 Free PMC article.
References
- Chardin P, Paris S, Antonny B, Robineau S, Béraud-Dufour S, Jackson C L, Chabre M. Nature (London) 1996;384:481–484. - PubMed
- Jackson C L, Casanova J E. Trends Cell Biol. 2000;10:60–67. - PubMed
- Cherfils J, Menetrey J, Mathieu M, Le Bras G, Robineau S, Béraud-Dufour S, Antonny B, Chardin P. Nature (London) 1998;392:101–105. - PubMed
- Mossessova E, Gulbis J M, Goldberg J. Cell. 1998;92:415–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources