NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions - PubMed (original) (raw)
NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions
A Chang et al. J Neurosci. 2000.
Abstract
Multiple sclerosis (MS) is characterized by multifocal loss of myelin, oligodendrocytes, and axons. Potential MS therapies include enhancement of remyelination by transplantation or manipulation of endogenous oligodendrocyte progenitor cells. Characteristics of endogenous oligodendrocyte progenitors in normal human brain and in MS lesions have not been studied extensively. This report describes the distribution of cells in sections from normal adult human brain and MS lesions by using antibodies directed against NG2, an integral membrane chondroitin sulfate proteoglycan expressed by oligodendrocyte progenitor cells. Stellate-shaped NG2-positive cells were detected in the white and gray matter of normal adult human brain and appeared as abundant as, but distinct from, astrocytes, oligodendrocytes, and microglia. Stellate-shaped or elongated NG2-positive cells also were detected in chronic MS lesions. A subpopulation of the elongated NG2-positive cells expressed the putative apoptotic signaling molecule p75(NTR). TUNEL-positive cells in three active, nine chronic active, and four chronic inactive lesions, however, were p75(NTR)-negative. These studies identify cells with phenotypic markers of endogenous oligodendrocyte progenitors in the mature human CNS and suggest that functional subpopulations of NG2-positive cells exist in MS lesions. Endogenous oligodendrocyte progenitor cells may represent a viable target for future therapies intended to enhance remyelination in MS patients.
Figures
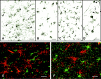
Fig. 1.
NG2 cells are abundant in the adult human brain and are distinct from microglia, oligodendrocytes, and astrocytes. NG2 antibodies stain relatively small perikarya, which emanate stellate-shaped processes that occupy discrete domains (A, arrowheads). NG2 is detected on endothelial cells also (A, arrows). PDGFαR antibodies stain cells with distributions identical to those of NG2 cells (B). PDGFαR is enriched in the cell bodies and in the larger processes. NG2 and PDGFαR cell morphology differs from MHC class II-stained microglia (C), which have shorter, thicker, and more irregularly shaped processes and from PLP-positive oligodendrocytes, which send processes (D, arrowheads) to myelin internodes (D, arrows). In double-labeled confocal images NG2 (E, F, red), LCA (E, green), and GFAP (F, green) antibodies stain different cell populations. Scale bars:A–C, 50 μm; D–F, 30 μm.
Fig. 2.
NG2 cells are detected in MS lesions and are distinct from microglia/monocytes and astrocytes. A–D, Artistic renditions of the distribution of NG2 cells in four MS lesions. The white areas are demyelinated periventricular (A, B) or subcortical (C, D) white matter, and the gray areas are myelinated white matter. NG2 cells (black cells) are distributed unevenly in the MS lesions (A–D). They have stellate (arrowheads) or elongated (arrows) shapes. ven, Ventricle;V, vessels; *, myelinated areas within a periventricular lesion. E, F, NG2-stained sections of MS lesions that contain stellate (E, arrowheads) or elongated (F) cells. Endothelial cells (E, arrow) are stained by NG2 antibodies also. Confocal images of MS lesions that have been double-labeled with NG2 (G, H, red), and the leukocyte/microglia marker LCA (G, green) or the astrocyte marker GFAP (H, green) have established that NG2 cells are distinct from leukocytes/microglia and astrocytes in MS lesions. MS case 1. Scale bars: E, 50 μm; F, 30 μm; G, H, 20 μm.
Fig. 3.
Some white matter MS lesions contain predominately stellate-shaped NG2 cells. The lighter area in_A_ demarcates a white matter MS lesion as identified by myelin staining in an adjacent section. Stellate-shaped NG2 cells (arrowheads) are present within the lesion. Blood vessels (arrows) are labeled also. In three separate MS lesions that contained predominately stellate-shaped NG2 cells, NG2 cell density within the lesion has been reduced significantly (p < 0.0001) when compared with NG2 cell density outside the lesion (B). MS case 2. Scale bar in A, 400 μm.
Fig. 4.
NG2 cell distribution appears unchanged in gray matter MS lesions. The distributions of myelin basic protein (A) and NG2 (B, C) are compared in adjacently cut sections from the cerebral cortex of an MS brain. The cerebral cortex (*, white area in A) is demyelinated with some remyelination. Subcortical white matter (black area in A) appears normally myelinated. The distribution and apparent density of NG2 cells (B) are not altered in demyelinated gray matter. The shape of NG2 cells in demyelinated gray matter (C, arrowheads) appears relatively normal. Arrows in_A_ and B demarcate the gray matter–white matter border. MS case 2. Scale bars: A, B, 200 μm;C, 50 μm.
Fig. 5.
Elongated NG2 and p75NTR-positive cells have similar distributions in MS lesions. Three serial sections are stained with MBP (A), NG2 (B), and p75NTR (C) antibodies.White areas in A represent demyelinated periventricular white matter. Elongated NG2 cells (B) and elongated p75NTR-positive cells (C) have similar distributions within the MS lesion. NG2 antibodies (B, arrowheads), but not p75NTR antibodies (C), have stained a population of stellate-shaped cells located subependymally. V, Vessel;ven, ventricle; *, myelinated fibers within the lesion. Most p75NTR-positive cells are elongated within the lesion (D, arrows) and at the edge of the lesion (E, arrows). However, small round p75NTR-positive cells also have been detected in normal-appearing white matter adjacent to MS lesions (E, arrowheads). MS case 1. Scale bars: A–C, 500 μm; D, E, 100 μm.
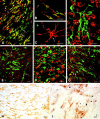
Fig. 6.
p75NTR is expressed by a subpopulation of NG2 cells in MS lesions. In confocal images that have been double-labeled with NG2 (A–C, red) and p75NTR (A–C, green) many, but not all, elongated cells express both antigens (A, B,yellow). Some elongated p75NTR-positive cells are NG2-negative (A, green, arrows), and some elongated NG2-positive cells are p75NTR-negative (A, B, red, arrowheads). Stellate-shaped NG2 cells (C, red, arrow) are not positive for p75NTR. In confocal images that have been double-labeled with p75NTR and LCA, p75NTR-positive cells (D, green) are distinct from leukocytes, macrophages, or microglia (D, red). Based on double labeling for p75NTR (E, green) and neurofilaments (E, red), elongated p75NTR-positive cells usually are oriented parallel to axons (E, arrows). p75NTR-positive elongated cells (F, green) have been detected in MS lesions that contain abundant MBP-positive myelin debris (F, red). p75NTR-positive elongated cells (G, green) often are enriched at the edge of MS lesions (G, red = MBP). TUNEL-positive cells (H, I, blue nuclei) are abundant in MS lesions. However, p75NTR-positive elongated or round cells are rarely TUNEL-positive (H, I). MS case 1. Scale bars:A, 50 μm; B–G, I, 20 μm;H, 100 μm.
Similar articles
- Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions.
Wilson HC, Scolding NJ, Raine CS. Wilson HC, et al. J Neuroimmunol. 2006 Jul;176(1-2):162-73. doi: 10.1016/j.jneuroim.2006.04.014. Epub 2006 Jun 6. J Neuroimmunol. 2006. PMID: 16753227 - The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS.
Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. Reynolds R, et al. J Neurocytol. 2002 Jul-Aug;31(6-7):523-36. doi: 10.1023/a:1025747832215. J Neurocytol. 2002. PMID: 14501221 - Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis.
Chang A, Tourtellotte WW, Rudick R, Trapp BD. Chang A, et al. N Engl J Med. 2002 Jan 17;346(3):165-73. doi: 10.1056/NEJMoa010994. N Engl J Med. 2002. PMID: 11796850 - NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system.
Polito A, Reynolds R. Polito A, et al. J Anat. 2005 Dec;207(6):707-16. doi: 10.1111/j.1469-7580.2005.00454.x. J Anat. 2005. PMID: 16367798 Free PMC article. Review. - NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors?
Dawson MR, Levine JM, Reynolds R. Dawson MR, et al. J Neurosci Res. 2000 Sep 1;61(5):471-9. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. J Neurosci Res. 2000. PMID: 10956416 Review.
Cited by
- Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin.
Ayoub R, Ruddy RM, Cox E, Oyefiade A, Derkach D, Laughlin S, Ades-Aron B, Shirzadi Z, Fieremans E, MacIntosh BJ, de Medeiros CB, Skocic J, Bouffet E, Miller FD, Morshead CM, Mabbott DJ. Ayoub R, et al. Nat Med. 2020 Aug;26(8):1285-1294. doi: 10.1038/s41591-020-0985-2. Epub 2020 Jul 27. Nat Med. 2020. PMID: 32719487 Free PMC article. Clinical Trial. - Macroglial diversity: white and grey areas and relevance to remyelination.
Werkman IL, Lentferink DH, Baron W. Werkman IL, et al. Cell Mol Life Sci. 2021 Jan;78(1):143-171. doi: 10.1007/s00018-020-03586-9. Epub 2020 Jul 9. Cell Mol Life Sci. 2021. PMID: 32648004 Free PMC article. Review. - The development of myelin repair agents for treatment of multiple sclerosis: progress and challenges.
Murphy RP, Murphy KJ, Pickering M. Murphy RP, et al. Bioengineered. 2013 May-Jun;4(3):140-6. doi: 10.4161/bioe.22835. Epub 2012 Nov 12. Bioengineered. 2013. PMID: 23147071 Free PMC article. Review. - CXCL12 Gene Therapy Ameliorates Ischemia-Induced White Matter Injury in Mouse Brain.
Li Y, Tang G, Liu Y, He X, Huang J, Lin X, Zhang Z, Yang GY, Wang Y. Li Y, et al. Stem Cells Transl Med. 2015 Oct;4(10):1122-30. doi: 10.5966/sctm.2015-0074. Epub 2015 Aug 7. Stem Cells Transl Med. 2015. PMID: 26253714 Free PMC article. - Cross-Talk of the CNS With Immune Cells and Functions in Health and Disease.
Matejuk A, Vandenbark AA, Offner H. Matejuk A, et al. Front Neurol. 2021 May 31;12:672455. doi: 10.3389/fneur.2021.672455. eCollection 2021. Front Neurol. 2021. PMID: 34135852 Free PMC article. Review.
References
- Althaus HH, Hempel R, Kloppner S, Engel J, Schmidt-Schultz T. Nerve growth factor signal transduction in mature pig oligodendrocytes. J Neurosci Res. 1997;50:729–742. - PubMed
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. - PubMed
- Bö L, Mörk S, Kong PA, Nyland H, Pardo CA, Trapp BD. Detection of MHC class II antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J Neuroimmunol. 1994;51:135–146. - PubMed
- Bonetti B, Raine CS. Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann Neurol. 1997;42:74–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS035058/NS/NINDS NIH HHS/United States
- P01 NS038667/NS/NINDS NIH HHS/United States
- P50 NS038667/NS/NINDS NIH HHS/United States
- F32 CA090073/CA/NCI NIH HHS/United States
- NS38667/NS/NINDS NIH HHS/United States
- NS35058/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials