Cerebral ischemia and seizures induce tyrosine phosphorylation of PYK2 in neurons and microglial cells - PubMed (original) (raw)
Cerebral ischemia and seizures induce tyrosine phosphorylation of PYK2 in neurons and microglial cells
D Tian et al. J Neurosci. 2000.
Abstract
The nonreceptor tyrosine kinase PYK2 represents a stress-sensitive mediator of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase (MAPK) signaling pathways in many cell types. In the present study, we assessed the tyrosine phosphorylation of PYK2 under normal and pathological conditions in the CNS. We generated a polyclonal antibody that selectively recognizes tyrosine-phosphorylated PYK2 at its major autophosphorylation site. By using this antibody, we demonstrate that the phosphorylation profile of PYK2 after focal cerebral ischemia is biphasic. The first phase occurs within 1 hr, when most of the phospho-PYK2 immunoreactivity was observed in cortical neurons, whereas 24-72 hr after ischemia, a striking induction of phospho-PYK2 immunoreactivity was evident in microglia around the necrotic infarcted area. Double-immunostaining analysis using both anti-phospho-PYK2 antibody and antibody against the double-phosphorylated active form of p38MAPK revealed that the two phosphorylated protein kinases exhibit strikingly similar distribution patterns after ischemia. A short time after ischemia, phosphorylation of p38MAPK was evident in the cortical neurons as demonstrated by both immunohistochemistry and immunoblotting analysis, whereas 24-72 hr after ischemia, phospho-p38MAPK was found in activated microglia and colocalized with phospho-PYK2. In contrast to cortical neurons, basal phospho-PYK2 immunoreactivity was observed in hippocampal pyramidal neurons, which was markedly decreased after kainate acid-induced status epilepticus. However, 24 hr after the epileptic onset, a pronounced upregulation of PYK2 and phospho-PYK2 immunoreactivities was evident in microglial cells, as demonstrated by double-immunostaining with the microglial marker OX42. These results provide, for the first time, in situ localization of tyrosine-phosphorylated PYK2 in neuronal stress pathways in the adult rat brain and are consistent with the role of PYK2 as an upstream regulator of p38MAPK signaling cascades in response to stress signals.
Figures
Fig. 1.
Specificity of anti-phospho PYK2 antibody by Western blot analysis. Shown are PYK2 immunoprecipitates from either quiescent PC12 cells or cells that were treated with 75 m
m
KCl for 10 min at 37°C. PYK2 immunoprecipitates were resolved on SDS-PAGE and analyzed by immunoblotting using anti-PYK2, anti-PTYR, or anti-phospho-PYK2 antibodies. Anti-PYK2 antibody recognizes PYK2 immunoprecipitated from both quiescent and KCl-stimulated PC12 cells (left), whereas anti-PTYR antibody recognizes PYK2, as well as several coimmunoprecipitated proteins only in response to KCl treatment. On the other hand, anti-phospho-PYK2 antibody recognizes only PYK2 immunoprecipitated from cells that were treated with KCl. No other tyrosine-phosphorylated proteins that coimmunoprecipitate with PYK2 are recognized by anti-phospho-PYK2 antibody (middle vs right).
Fig. 2.
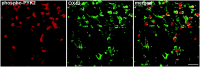
Enhanced phospho-PYK2 immunoreactivity in cortical neurons after focal cerebral ischemia. Coronal brain sections (50 μm) of animals killed at the indicated time points after MCAO were double-immunostained with anti-PTYR monoclonal antibody and with either anti-PYK2 (left) or anti-phospho-PYK2 (right) polyclonal antibodies. Shown are confocal images of lamina V neurons within the infarct cortex. In the control sections, a strong PYK2 immunoreactivity was visualized, whereas no detectable immunoreactivity of either phospho-PYK2 or PTYR was detected. At 15 min after ischemia, intense phospho-PYK2 and PTYR immunoreactivities were observed in the neurons, which were further increased at 30 min and slightly decreased at 1 hr. PTYR-positive microglial cells (arrows) were observed 1 hr after MCAO. Scale bar, 50 μm.
Fig. 3.
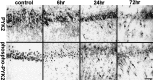
Immunoreactivity of phospho-PYK2 and OX42 within the core infract (striatum) after focal cerebral ischemia. Coronal brain sections (50 μm) of animals killed at the indicated time points after MCAO were immunostained with either anti-OX42 (top row) or anti-phospho-PYK2 (bottom row) antibody. No detectable immunoreactivity of phospho-PYK2 was observed in the control sections, whereas OX42-positive resting microglial cells were randomly distributed in the striatum. At 6 hr after MCAO, phospho-PYK2 immunoreactivity was visualized in microglia-like cells in the affected striatum. Note that the morphology of phospho-PYK2-positive cells resembles the microglia, as demonstrated by OX42 immunostaining. A further increase in phospho-PYK2 immunoreactivity was observed over 24–72 hr after MCAO. The number of microglial cells and their morphology were dramatically changed during this period, as demonstrated by OX42 immunostaining. By 72 hr, the core infarction area was filled with amoeboid-like microglial cells. Scale bar, 50 μm.
Fig. 4.
Colocalization of phospho-PYK2 and OX42 in microglial cells after focal cerebral ischemia. Coronal brain sections (50 μm) of animals killed at 72 hr after MCAO were double-immunostained with anti-phospho-PYK2 and anti-OX42 antibodies. Shown are confocal images of phospho-PYK2 (red) and OX42 (green) immunostaining and the merged image. Colocalization is shown in yellow. As demonstrated, phospho-PYK2 appears mainly in the cytosol, whereas OX42 appears in the cell periphery. Scale bar, 25 μm.
Fig. 5.
Colocalization of phospho-PYK2 and phospho-p38MAPK in cortical neurons after focal cerebral ischemia. Coronal brain sections (50 μm) of animals killed at the indicated time points after MCAO were double-immunostained with anti-phospho-p38MAPK monoclonal antibody and anti-phospho-PYK2 polyclonal antibody. Shown are confocal images of phospho-PYK2 (red) and phospho-p38MAPK (green) immunoreactivity in lamina V neurons of the cortex. Phospho-PYK2 and p38MAPK were barely detectable in the control cortex, whereas intense phospho-PYK2 and p38MAPK immunoreactivities were visualized in the cortex at 15 min after ischemia. These immunoreactivities were further increased at 30 min. Scale bar, 50 μm.
Fig. 6.
Increased phosphorylation of PYK2 and p38MAPK in the cortex after ischemia. Shown are PYK2 and p38MAPK immunoprecipitates prepared from homogenates of either control animals or animals that were exposed to MCAO for the indicated time. PYK2 and p38MAPK immunoprecipitates were resolved on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-PYK2, anti-phospho-PYK2, anti-p38MAPK, or anti-phospho-p38MAPK antibodies. An increase in PYK2 and p38MAPK phosphorylation was observed in the cortex at 15 min after MCAO (top row). The phosphorylation of PYK2 was sustained for 1 hr after MCAO and dramatically decreased at 6 hr, whereas slight decrease of p38MAPK phosphorylation was evident 6 hr after MCAO. No detectable changes of either PYK2 or p38MAPK expression levels was observed during the first 6 hr after MCAO (bottom row).
Fig. 7.
Colocalization of phospho-PYK2 and phospho-p38MAPK in microglial cells after focal cerebral ischemia. Coronal brain sections (50 μm) of animals killed at 72 hr after MCAO were double-immunostained with anti-phospho-p38MAPK monoclonal antibody and anti-phospho-PYK2 polyclonal antibody. Shown are confocal images of phospho-PYK2 and phospho-p38MAPK immunoreactivities within the infarct striatum in microglial cells 72 hr after ischemia. Colocalization appears in yellow. As shown, the amoeboid-like microglial cells are double-immunostained with anti-phospho-PYK2 and anti-phospho-p38MAPK antibodies. Scale bar, 50 μm.
Fig. 8.
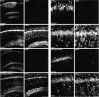
Tyrosine phosphorylation of PYK2 within the hippocampus after KA-induced epilepsy. Coronal brain sections (50 μm) of animals killed at the indicated time points were immunostained with either anti-PYK2 (top row) or with anti-phospho-PYK2 (bottom row) antibody. Shown are photomicrographs of the hippocampal CA1 field. Intense PYK2 and moderate phospho-PYK2 immunoreactivity were detected in the CA1 pyramidal neurons of the control hippocampus. PYK2 and phospho-PYK2 immunoreactivities were dramatically decreased in the CA1 pyramidal neurons 6 hr after epileptic onset and were barely detected at 72 hr. However, PYK2 and phospho-PYK2 immunoreactivities were evident in microglia-like cells at 24–72 hr after KA injection (arrows). At 72 hr after KA injection, pronounced immunoreactivity of PYK2 and phospho-PYK2 was observed in rod-like microglial cells. Scale bar, 50 μm.
Fig. 9.
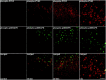
Colocalization of phospho-PYK2 and OX42 in the microglial cells after KA-induced epilepsy. Coronal brain sections (50 μm) of either control animals or animals killed at 72 hr after epileptic onset were double-immunostained with anti-OX42 monoclonal antibody and with either anti-PYK2 or anti-phospho-PYK2 polyclonal antibody. As shown in low-power confocal images (left panel), intense PYK2 and moderate phospho-PYK2 immunoreactivities were visualized in CA1 pyramidal neurons and granule cells of the dentate gyrus in the control hippocampus. Microglial cells immunostained with OX42 are uniformly distributed throughout the control hippocampus, and no colocalization with either PYK2 or phospho-PYK2 was observed. At 72 hr after seizure, OX42-positive microglial cells were accumulated in the CA1 pyramidal layer, striatum radiatum, and dentate hilus. Enhanced PYK2 and phospho-PYK2 immunoreactivities were observed in microglial cells 72 hr after seizure. High-power confocal images (right panel) demonstrate the dramatic changes in PYK2, phospho-PYK2, and OX42 immunoreactivities in the CA1 field. At 72 hr, most of the OX42-positive rod-like microglial cells (arrows) exhibit intense PYK2 and phospho-PYK2 immunoreactivity. Scale bars:left, 200 μm; right, 50 μm.
Similar articles
- Protein-tyrosine kinase Pyk2 mediates endothelin-induced p38 MAPK activation in glomerular mesangial cells.
Sorokin A, Kozlowski P, Graves L, Philip A. Sorokin A, et al. J Biol Chem. 2001 Jun 15;276(24):21521-8. doi: 10.1074/jbc.M008869200. Epub 2001 Feb 26. J Biol Chem. 2001. PMID: 11278444 - Activation of p38MAPK in microglia after ischemia.
Walton KM, DiRocco R, Bartlett BA, Koury E, Marcy VR, Jarvis B, Schaefer EM, Bhat RV. Walton KM, et al. J Neurochem. 1998 Apr;70(4):1764-7. doi: 10.1046/j.1471-4159.1998.70041764.x. J Neurochem. 1998. PMID: 9523596 - Antidepressant effect of the calcium-activated tyrosine kinase Pyk2 in the lateral septum.
Sheehan TP, Neve RL, Duman RS, Russell DS. Sheehan TP, et al. Biol Psychiatry. 2003 Sep 1;54(5):540-51. doi: 10.1016/s0006-3223(02)01815-2. Biol Psychiatry. 2003. PMID: 12946883 - The Non-receptor Tyrosine Kinase Pyk2 in Brain Function and Neurological and Psychiatric Diseases.
de Pins B, Mendes T, Giralt A, Girault JA. de Pins B, et al. Front Synaptic Neurosci. 2021 Oct 6;13:749001. doi: 10.3389/fnsyn.2021.749001. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34690733 Free PMC article. Review.
Cited by
- Mitogen-activated protein kinase phosphatase-1 (MKP-1) in retinal ischemic preconditioning.
Dreixler JC, Bratton A, Du E, Shaikh AR, Savoie B, Alexander M, Marcet MM, Roth S. Dreixler JC, et al. Exp Eye Res. 2011 Oct;93(4):340-9. doi: 10.1016/j.exer.2010.10.011. Epub 2010 Nov 20. Exp Eye Res. 2011. PMID: 21094639 Free PMC article. - Transient Receptor Potential-Melastatin Channel Family Member 2: Friend or Foe.
Cheung JY, Miller BA. Cheung JY, et al. Trans Am Clin Climatol Assoc. 2017;128:308-329. Trans Am Clin Climatol Assoc. 2017. PMID: 28790515 Free PMC article. Review. - Microglia Activate Migration of Glioma Cells through a Pyk2 Intracellular Pathway.
Rolón-Reyes K, Kucheryavykh YV, Cubano LA, Inyushin M, Skatchkov SN, Eaton MJ, Harrison JK, Kucheryavykh LY. Rolón-Reyes K, et al. PLoS One. 2015 Jun 22;10(6):e0131059. doi: 10.1371/journal.pone.0131059. eCollection 2015. PLoS One. 2015. PMID: 26098895 Free PMC article. - Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia.
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Tikka T, et al. J Neurosci. 2001 Apr 15;21(8):2580-8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. J Neurosci. 2001. PMID: 11306611 Free PMC article. - RNA-seq expression profiling of rat MCAO model following reperfusion Orexin-A.
Wang CM, Pan YY, Liu MH, Cheng BH, Bai B, Chen J. Wang CM, et al. Oncotarget. 2017 Dec 6;8(68):113066-113081. doi: 10.18632/oncotarget.22995. eCollection 2017 Dec 22. Oncotarget. 2017. PMID: 29348887 Free PMC article.
References
- Arias RL, Tasse JR, Bowlby MR. Neuroprotective interaction effects of NMDA and AMPA receptor antagonists in an in vitro model of cerebral ischemia. Brain Res. 1999;816:299–308. - PubMed
- Behrens MM, Strasser U, Koh JY, Gwag BJ, Choi DW. Prevention of neuronal apoptosis by phorbol ester-induced activation of protein kinase C: blockade of p38 mitogen-activated protein kinase. Neuroscience. 1999;94:917–927. - PubMed
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. - PubMed
- Berg NN, Ostergaard HL. T cell receptor engagement induces tyrosine phosphorylation of FAK and Pyk2 and their association with Lck. J Immunol. 1997;159:1753–1757. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous