Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in A. baumannii is not due solely to the presence of beta-lactamases - PubMed (original) (raw)
Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in A. baumannii is not due solely to the presence of beta-lactamases
G Bou et al. J Clin Microbiol. 2000 Sep.
Abstract
From February to November 1997, 29 inpatients at Ramón y Cajal Hospital, Madrid, Spain, were determined to be either colonized or infected with imipenem- and meropenem-resistant Acinetobacter baumannii (IMRAB) strains (MICs, 128 to 256 microg/ml). A wide antibiotic multiresistance profile was observed with IMRAB strains. For typing IMRAB isolates, pulsed-field gel electrophoresis was used. For comparative purposes, 30 imipenem- and meropenem-susceptible A. baumannii (IMSAB) strains isolated before, during, and after the outbreak were included in this study. The molecular-typing results showed that the outbreak was caused by a single IMRAB strain (genotype A). By cloning experiments we identified a class D beta-lactamase (OXA-24) encoded in the chromosomal DNA of this IMRAB strain which showed carbapenem hydrolysis. Moreover, the outer membrane profile of the IMRAB strain showed a reduction in the expression of two porins at 22 and 33 kDa when compared with genetically related IMSAB isolates. In addition no efflux mechanisms were identified in the IMRAB strains. In summary, we report here the molecular characterization of a nosocomial outbreak caused by one multiresistant A. baumannii epidemic strain that harbors a carbapenem-hydrolyzing enzyme. Although alterations in the penicillin-binding proteins cannot be ruled out, the reduction in the expression of two porins and the presence of this OXA-derived beta-lactamase are involved in the carbapenem resistance of the epidemic nosocomial IMRAB strain.
Figures
FIG. 1
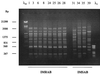
Patterns obtained by PFGE with _Sma_I. Lanes 1, 10, 14, and 19, low-range PFG DNA marker (New England Biolabs, United Kingdom). Lanes 2 through 9, IMRAB strains 1, 3, 6, 8, 24, 25, 26, and 28, respectively, in Table 1. Lanes 11 through 13, IMSAB PRE strains 31 to 33, respectively; lanes 15 through 18, IMSAB AT strains 34 through 37, respectively; lanes 20 through 22, IMSAB POST strains 38 through 40, respectively.
FIG. 2
Patterns obtained by REP-PCR. The numbers above the figure indicate the corresponding strains (Table 1). Lanes λIII and λV, DNA molecular weight markers III and V, respectively (Boehringer GmbH, Mannheim, Germany).
FIG. 3
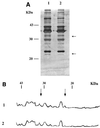
(A) OMP profile of the IMSAB strain 34 (Table 1) (lane 1) and IMRAB strain RYC 52763/97 (lane 2). SDS—12.5% polyacrylamide gel electrophoresis and silver staining. Arrows, the different expression of porins at 22 and 33 kDa. (B) Densitometric analysis of the gel shown in panel A. 1, IMSAB protein pattern; 2, IMRAB protein pattern. Arrows indicate the different expression of porins at 22 and 33 kDa.
FIG. 4
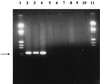
Absence of the OXA-24 gene in different carbapenem-susceptible IMSAB strains by PCR amplification with P1 and P2 OXA primers. Lanes 1 and 11, molecular weight marker λIII (Boehringer); lane 2, A. baumannii RYC 52763/97 as the positive control; lanes 3 and 4, two epidemic genetically related IMRAB strains; lanes 5 through 10, different genetically unrelated carbapenem-susceptible IMSAB strains.
Similar articles
- PCR-based DNA fingerprinting (REP-PCR, AP-PCR) and pulsed-field gel electrophoresis characterization of a nosocomial outbreak caused by imipenem- and meropenem-resistant Acinetobacter baumannii.
Bou G, Cerveró G, Domínguez MA, Quereda C, Martínez-Beltrán J. Bou G, et al. Clin Microbiol Infect. 2000 Dec;6(12):635-43. doi: 10.1046/j.1469-0691.2000.00181.x. Clin Microbiol Infect. 2000. PMID: 11284921 - Molecular mechanisms associated with nosocomial carbapenem-resistant Acinetobacter baumannii in Mexico.
Alcántar-Curiel MD, García-Torres LF, González-Chávez MI, Morfín-Otero R, Gayosso-Vázquez C, Jarillo-Quijada MD, Fernández-Vázquez JL, Giono-Cerezo S, Rodríguez-Noriega E, Santos-Preciado JI. Alcántar-Curiel MD, et al. Arch Med Res. 2014 Oct;45(7):553-60. doi: 10.1016/j.arcmed.2014.10.006. Epub 2014 Nov 1. Arch Med Res. 2014. PMID: 25450581 - A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA-58.
Héritier C, Dubouix A, Poirel L, Marty N, Nordmann P. Héritier C, et al. J Antimicrob Chemother. 2005 Jan;55(1):115-8. doi: 10.1093/jac/dkh500. Epub 2004 Dec 8. J Antimicrob Chemother. 2005. PMID: 15590718 - [The high level resistance to carbapenems in Acinetobacter baumannii is a multifactorial problem].
Bou G. Bou G. Enferm Infecc Microbiol Clin. 2001 Aug-Sep;19(7):336-8. doi: 10.1016/s0213-005x(01)72653-5. Enferm Infecc Microbiol Clin. 2001. PMID: 11747791 Review. Spanish. No abstract available. - Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology.
Poirel L, Nordmann P. Poirel L, et al. Clin Microbiol Infect. 2006 Sep;12(9):826-36. doi: 10.1111/j.1469-0691.2006.01456.x. Clin Microbiol Infect. 2006. PMID: 16882287 Review.
Cited by
- Acinetobacter Baumannii Phages: Past, Present and Future.
Tu Q, Pu M, Li Y, Wang Y, Li M, Song L, Li M, An X, Fan H, Tong Y. Tu Q, et al. Viruses. 2023 Mar 3;15(3):673. doi: 10.3390/v15030673. Viruses. 2023. PMID: 36992382 Free PMC article. Review. - Whole-Genome Sequencing-Based Resistome Analysis of Nosocomial Multidrug-Resistant Non-Fermenting Gram-Negative Pathogens from the Balkans.
Peykov S, Strateva T. Peykov S, et al. Microorganisms. 2023 Mar 3;11(3):651. doi: 10.3390/microorganisms11030651. Microorganisms. 2023. PMID: 36985224 Free PMC article. Review. - Virulence Potential and Treatment Options of Multidrug-Resistant (MDR) Acinetobacter baumannii.
Kumar S, Anwer R, Azzi A. Kumar S, et al. Microorganisms. 2021 Oct 6;9(10):2104. doi: 10.3390/microorganisms9102104. Microorganisms. 2021. PMID: 34683425 Free PMC article. Review. - An Evaluation of BfmR-Regulated Antimicrobial Resistance in the Extensively Drug Resistant (XDR) Acinetobacter baumannii Strain HUMC1.
Marr CM, MacDonald U, Trivedi G, Chakravorty S, Russo TA. Marr CM, et al. Front Microbiol. 2020 Oct 29;11:595798. doi: 10.3389/fmicb.2020.595798. eCollection 2020. Front Microbiol. 2020. PMID: 33193275 Free PMC article. - Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii.
Vrancianu CO, Gheorghe I, Czobor IB, Chifiriuc MC. Vrancianu CO, et al. Microorganisms. 2020 Jun 21;8(6):935. doi: 10.3390/microorganisms8060935. Microorganisms. 2020. PMID: 32575913 Free PMC article. Review.
References
- Afzal M S, Livermore D. Worldwide emergence of carbapemem-resistant Acinetobacter spp. J Antimicrob Chemother. 1998;41:576–577. - PubMed
- Amyes S G B, Young H-K. Mechanism of antibiotic resistance in Acinetobacter spp.—genetics of resistance. In: Bergogne-Berezin E, Joly-Guillou M L, Towner K J, editors. Acinetobacter: microbiology, epidemiology, infections, management, 1996. New York, N.Y: CRC Press; 1996. pp. 185–223.
- Bergogne-Bérézin E. Resistance of Acinetobacter spp. to antimicrobials—overview of clinical resistance patterns and therapeutic problems. In: Bergogne-Berezin E, Joly-Guillou M L, Towner K J, editors. Acinetobacter: microbiology, epidemiology, infections, management, 1996. New York, N.Y: CRC Press; 1996. pp. 133–183.
- Bergogne-Bérézin E, Joly-Guillou M L, Vieu J F. Epidemiology of nosocomial infections due to Acinetobacter calcoaceticus. J Hosp Infect. 1987;10:105–113. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases