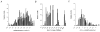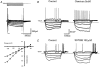Electrophysiological and morphological characteristics of three subtypes of rat globus pallidus neurone in vitro - PubMed (original) (raw)
Electrophysiological and morphological characteristics of three subtypes of rat globus pallidus neurone in vitro
A J Cooper et al. J Physiol. 2000.
Abstract
Neurones of the globus pallidus (GP) have been classified into three subgroups based on the visual inspection of current clamp electrophysiological properties and morphology of biocytin-filled neurones. Type A neurones (132/208; 63 %) were identified by the presence of the time- and voltage-dependent inward rectifier (Ih) and the low-threshold calcium current (It) giving rise to anodal break depolarisations. These cells were quiescent or fired regular spontaneous action potentials followed by biphasic AHPs. Current injection evoked regular activity up to maximum firing frequency of 350 Hz followed by moderate spike frequency adaptation. The somata of type A cells were variable in shape (20 x 12 micrometer) while their dendrites were highly varicose. Type B neurones (66/208; 32 %) exhibited neither Ih nor rebound depolarisations and only a fast monophasic AHP. These cells were spontaneously active while current injection induced irregular patterns of action potential firing up to a frequency of 440 Hz with weak spike frequency adaptation. Morphologically, these cells were the smallest encountered (15 x 10 micrometer), oval in shape with restricted varicose dendritic arborisations. Type C neurones were much rarer (10/208; 5 %). They were identified by the absence of Ih and rebound depolarisations, but did possess a prolonged biphasic AHP. They displayed large A-like potassium currents and ramp-like depolarisations in response to step current injections, which induced firing up to a maximum firing frequency of 310 Hz. These cells were the largest observed (27 x 15 micrometer) with extensive dendritic branching. These results confirm neuronal heterogeneity in the adult rodent GP. The driven activity and population percentage of the three subtypes correlates well with the in vivo studies (Kita & Kitai, 1991). Type A cells appear to correspond to type II neurones of Nambu & Llinas (1994, 1997) while the small diameter type B cells display morphological similarities with those described by Millhouse (1986). The rarely encountered type C cells may well be large cholinergic neurones. These findings provide a cellular basis for the study of intercellular communication and network interactions in the adult rat in vitro.
Figures
Figure 1. Frequency distribution histograms of membrane characteristics are indicative of a heterogeneous population of GP neurones
Of 296 cells recorded in the GP 208 (70 %) have been classified into three subtypes (filled bars). Open bars indicate unclassified cells. Frequency distribution histograms of resting membrane potential (A), input resistance (B) and action potential duration (C). None of these parameters exhibited a Gaussian distribution expected of a homogenous population of neurones indicative of two or more cell types in the GP.
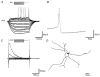
Figure 2. Type A GP neurones
A, records of membrane potential in response to a series of 300 ms current steps (in 25 pA increments) from resting membrane potential. Large hyperpolarising current steps elicit a characteristic time- and voltage-dependent ‘sag’ in membrane potential. Rebound depolarisations often accompanied by action potential firing were produced on repolarisation of the membrane. B, a representative single action potential of duration 1.03 ms followed by long lasting after-hyperpolarisation. C, records of membrane current in response to a series of 300 ms steps (10 mV increments) from a holding potential of −60 mV showing time- and voltage-dependent inward current evoked on hyperpolarising voltage steps. D, camera lucida drawing of type A multipolar GP neurone with varicose dendrites following intracellular labelling with biocytin.
Figure 3. Type A cells show anomalous time- and voltage-dependent inward rectification (_I_h)
A, superimposed membrane currents in response to a series of 200 ms voltage steps, in 10 mV increments, from −120 to −40 mV from a holding potential of −60 mV. Hyperpolarising steps evoke a slow time- and voltage-dependent inward current (_I_h). The instantaneous current (•, ins) and steady state current (▴, ss) are plotted in current-voltage relationship below showing activation at potentials less than −70 mV. B, records of membrane potential in response to a series of current steps showing pronounced sag of membrane voltage due to _I_h. Bath application of caesium chloride (2 m
m
) increases the membrane resistance and abolishes the sag in membrane voltage. C, records of membrane potential from a different cell showing that bath application of ZD7288 (100 μM) a specific blocker of _I_h also abolishes the sag in membrane voltage.
Figure 4. Type A cells have a low threshold calcium conductance (_I_t)
A, upper panel: superimposed leak subtracted (P/4) membrane currents from a type A cell in response to voltage steps (increments of 2.5 mV beginning at −70 mV), from a holding potential of −100 mV in the presence of TTX (1 μM) and TEA-Cl (20 m
m
). Lower panel: current-voltage relationship for the inward currents shows activation at −57.5 mV reaching a peak at −47.5 mV. Residual TEA-Cl insensitive outward currents are evoked at more depolarised step potentials. B, leak subtracted (P/4) currents evoked as in A, showing control (a) and the effect of bath application of NiCl2 (1 m
m
; b). The inward current is abolished, while the outward component also appears reduced by NiCl2_C_, voltage records in response to 200 pA hyperpolarising steps in control (a) and upon bath application of NiCl2 (1 m
m
; b). Cc shows a comparison of a and b highlighting the reduction in amplitude of the rebound depolarisation and abolition of action potential firing.
Figure 5. Type B GP neurones
A, records of membrane potential in response to a series of 300 ms hyperpolarising current steps (in 25 pA increments), from resting membrane potential showing no evidence for ‘sag’ in membrane potential and no rebound depolarisations. Note the spontaneous activity of this cell. B, a representative single action potential showing action potential duration of 0.68 ms followed by a short lasting (fast) after-hyperpolarisation. C, records of membrane current in response to a series of 300 ms steps (10 mV increments) from a holding potential of −60 mV showing little evidence for _I_h. On depolarising steps there is evidence of an inward current and both transient and sustained outward currents. D, camera lucida drawings of a typically small type B GP neurone with sparse dendritic tree, following intracellular labelling with biocytin.
Figure 6. Type C neurones
A, records of membrane potential in response to a series of 300 ms current steps (in 25 pA increments), from resting membrane potential. Note the resting membrane potential of this quiescent cell, long time constant of membrane charging, the lack of _I_h and rebound depolarisations. B, a representative single action potential showing action potential duration of 0.95 ms followed by a prolonged after-hyperpolarisation. C, records of membrane current in response to a series of 300 ms steps (10 mV increments) from a holding potential of −60 mV. There is no evidence of _I_h on hyperpolarising voltage steps. A large transient outward current was observed on depolarising steps indicative of _I_A. D, camera lucida drawings of a typical large pyramidal, type C GP neurone following intracellular labelling with biocytin.
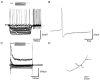
Figure 7. Driven activity of GP cells
Depolarising current steps of 1000 ms duration were applied from resting membrane potential. Representative voltage records are given for each cell type, showing minimal current required to elicit action potential firing. Note the regular firing of type A cell, the irregular firing of type B cells and the ramp-like depolarisation of type C cell. Instantaneous frequencies against time during current step, for a series of step depolarisations are shown below. B-type cells can sustain highest firing frequency although this rate is very irregular. Spike frequency adaptation increases with the size of the current step in both A and B cells. ▪, frequency of activity immediately prior to spike accommodation.
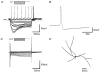
Figure 8. Morphological reconstruction of biocytin-labelled neurones
Of the 61 cells that were sufficiently well labelled with biocytin, 40 have been fully characterised electrophysiologically. A, examples of large multipolar type A neurones with extensive dendritic branching, which were mainly varicose (21/26 cells). B, representative examples of small oval type B GP cells whose dendrites were predominantly varicose (7/10 cells). C, examples of large pyramidal type C cells with extensive dendritic trees.
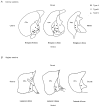
Figure 9. Topography of GP cells
The location of each biocytin-filled GP neurone was plotted onto stylised drawings of coronal (A) and sagittal (B) slices (obtained from Paxinos & Watson 1986). There appears to be a homogenous distribution of neuronal types A, B and C throughout the GP.
Similar articles
- Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus.
Pennartz CM, De Jeu MT, Geurtsen AM, Sluiter AA, Hermes ML. Pennartz CM, et al. J Physiol. 1998 Feb 1;506 ( Pt 3)(Pt 3):775-93. doi: 10.1111/j.1469-7793.1998.775bv.x. J Physiol. 1998. PMID: 9503337 Free PMC article. - Morphology and membrane properties of neurones in the cat ventrobasal thalamus in vitro.
Turner JP, Anderson CM, Williams SR, Crunelli V. Turner JP, et al. J Physiol. 1997 Dec 15;505 ( Pt 3)(Pt 3):707-26. doi: 10.1111/j.1469-7793.1997.707ba.x. J Physiol. 1997. PMID: 9457647 Free PMC article. - Membrane properties and morphology of vasopressin neurons in slices of rat suprachiasmatic nucleus.
Pennartz CM, Bos NP, Jeu MT, Geurtsen AM, Mirmiran M, Sluiter AA, Buijs RM. Pennartz CM, et al. J Neurophysiol. 1998 Nov;80(5):2710-7. doi: 10.1152/jn.1998.80.5.2710. J Neurophysiol. 1998. PMID: 9819275 - Phenotypic and state-dependent expression of the electrical and morphological properties of oxytocin and vasopressin neurones.
Armstrong WE, Stern JE. Armstrong WE, et al. Prog Brain Res. 1998;119:101-13. doi: 10.1016/s0079-6123(08)61564-2. Prog Brain Res. 1998. PMID: 10074783 Review.
Cited by
- Synaptic Changes in Pallidostriatal Circuits Observed in the Parkinsonian Model Triggers Abnormal Beta Synchrony with Accurate Spatio-temporal Properties across the Basal Ganglia.
Azizpour Lindi S, Mallet NP, Leblois A. Azizpour Lindi S, et al. J Neurosci. 2024 Feb 28;44(9):e0419232023. doi: 10.1523/JNEUROSCI.0419-23.2023. J Neurosci. 2024. PMID: 38123981 Free PMC article. - Electrophysiological characteristics of globus pallidus neurons.
Bugaysen J, Bronfeld M, Tischler H, Bar-Gad I, Korngreen A. Bugaysen J, et al. PLoS One. 2010 Aug 6;5(8):e12001. doi: 10.1371/journal.pone.0012001. PLoS One. 2010. PMID: 20700458 Free PMC article. - Short-term plasticity shapes activity pattern-dependent striato-pallidal synaptic transmission.
Kim J, Kita H. Kim J, et al. J Neurophysiol. 2013 Feb;109(4):932-9. doi: 10.1152/jn.00459.2012. Epub 2012 Nov 28. J Neurophysiol. 2013. PMID: 23197459 Free PMC article. - Modulation of in vivo GABA-evoked responses by nitric oxide-active compounds in the globus pallidus of rat.
Carletti F, Ferraro G, Rizzo V, Friscia S, Sardo P. Carletti F, et al. J Neural Transm (Vienna). 2012 Aug;119(8):911-21. doi: 10.1007/s00702-011-0760-0. Epub 2012 Jan 19. J Neural Transm (Vienna). 2012. PMID: 22258796 - Firing rate and pattern heterogeneity in the globus pallidus arise from a single neuronal population.
Deister CA, Dodla R, Barraza D, Kita H, Wilson CJ. Deister CA, et al. J Neurophysiol. 2013 Jan;109(2):497-506. doi: 10.1152/jn.00677.2012. Epub 2012 Oct 31. J Neurophysiol. 2013. PMID: 23114208 Free PMC article.
References
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltranferase. Journal of Comparative Neurology. 1983;216:53–68. - PubMed
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and Parkinsonian primates. Trends in Neurosciences. 1998;21:32–38. - PubMed
- Bolam JP, Smith Y. The striatum and globus pallidus send convergent synaptic inputs onto single cells in the entopeduncular nucleus of the rat: a double antereograde labelling study combined with post-embedding immunocytochemistry for GABA. Journal of Comparative Neurology. 1992;321:456–476. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources