Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway - PubMed (original) (raw)
Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway
D C Raitt et al. EMBO J. 2000.
Abstract
The adaptive response to hyperosmotic stress in yeast, termed the high osmolarity glycerol (HOG) response, is mediated by two independent upstream pathways that converge on the Pbs2 MAP kinase kinase (MAPKK), leading to the activation of the Hog1 MAP kinase. One branch is dependent on the Sho1 transmembrane protein, whose primary role was found to be the binding and translocation of the Pbs2 MAPKK to the plasma membrane, and specifically to sites of polarized growth. The yeast PAK homolog Ste20 is essential for the Sho1-dependent activation of the Hog1 MAP kinase in response to severe osmotic stress. This function of Ste20 in the HOG pathway requires binding of the small GTPase Cdc42. Overexpression of Cdc42 partially complements the osmosensitivity of ste20Delta mutants, perhaps by activating another PAK-like kinase, while a dominant-negative Cdc42 mutant inhibited signaling through the SHO1 branch of the HOG pathway. Since activated Cdc42 translocates Ste20 to sites of polarized growth, the upstream and downstream elements of the HOG pathway are brought together through the membrane targeting function of Sho1 and Cdc42.
Figures
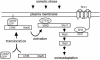
Fig. 1. Schematic model outlining the role of Sho1, Cdc42 and Ste20 in the HOG MAP kinase pathway. The membrane-bound protein Sho1 anchors the Hog1 MAP kinase module to the membrane through its interaction with the Pbs2 MAPKK, which in turn interacts with the Ste11 MAPKKK and Hog1 MAP kinase. In response to osmotic stress, Cdc42-bound, membrane-localized Ste20 phosphorylates Ste11, which leads to the activation of the Pbs2 MAPKK and the Hog1 MAP kinase, and eventually to the osmo-adaptive response. Exactly how osmotic stress induces Ste20 activation is unclear. 50BD, Ste50 binding domain; CRIB, Cdc42/Rac interactive binding domain; PPP, proline-rich sequence.
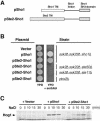
Fig. 2. A chimeric protein composed of the transmembrane domains of Ste2 and the cytoplasmic domain of Sho1 is functional. (A) Schematic representation of the wild-type Sho1 and the Ste2–Sho1 chimeric constructs. A region of the STE2 gene encoding the seven transmembrane (TM) domains was fused to a region of the SHO1 gene encoding the cytoplasmic linker region and the SH3 domain (residues 146–367). (B) Complementation of sho1Δ mutation by the Ste2–Sho1 chimera. Yeast strains MY007 (ssk2Δ ssk22Δ sho1Δ), FP67 (ssk2Δ ssk22Δ ste50Δ), FP50 (ssk2Δ ssk22Δ ste11Δ) and TM260 (pbs2Δ) were transformed with wild-type SHO1 (pSho1), the STE2–SHO1 chimeric construct (pSte2_–_Sho1), or the empty vector pYES2 (Vector). Rescue of the osmosensitive phenotype of this strain was assessed by spotting transformed cells onto YPD plates containing 1.5 M sorbitol. Although the Ste2_–_Sho1 chimeric protein could functionally substitute for wild-type Sho1, it failed to complement the osmosensitive defect of ste50Δ, ste11Δ and pbs2Δ strains (ssk2Δ and ssk22Δ mutations are included in some strains to inactivate the SLN1 branch of the HOG pathway). (C) The Ste2_–_Sho1 chimera can activate the Hog1 MAP kinase with kinetics similar to wild-type Sho1 protein in response to external osmotic stress. The same transformants as in (B) were tested by 4G10 immunoblot assay to detect phosphorylation of the Hog1 MAP kinase before (time 0), and 5, 10, 15 and 30 min after the addition of 0.4 M NaCl.
Fig. 3. Membrane localization of Sho1 is necessary and sufficient for its role in the osmotic stress response. (A) Osmosensitivity of sho1Δ mutant transformed with the indicated plasmids. pMyr_–_Sho1 encodes a variant of Sho1 that lacks the four transmembrane domains but does contain a membrane-targeting myristoylation site. pMyrAS_–_Sho1 is a derivative of pMyr_–_Sho1 with a myristoylation-defective mutation. These constructs were placed under control of the inducible galactose promoter in the pYES2 vector, and used to transform the yeast strain MY007 (ssk2Δ ssk22Δ sho1Δ). Thus, expression of the Myr_–_Sho1 constructs is suppressed on glucose media (YPD) and induced on galactose media (YPGal). Expression of the membrane-targeting Myr_–_Sho1 construct, but not that of the mutant MyrAS_–_Sho1, complemented the osmosensitive defect of sho1Δ mutation. (B) Tyrosine phosphorylation of Hog1 following the addition of 0.4 M NaCl. The same strains used in (A) were subjected to immunoblot analysis using anti-phosphotyrosine antibody 4G10.
Fig. 4. Requirement for the Sho1 cytoplasmic linker region and the SH3 domain for Sho1 function in the osmotic stress response. (A) Schematic representation of the Sho1 constructs used in this experiment. The pSte2_–_Sho1 chimeric construct is described in Figure 2. Its derivatives, pSte2_–_Sho1-A and pSte2_–_Sho1-B, contain deletions within the Sho1 linker region. In pSte2_–_Sho1-C, the Sho1 linker region is replaced with an unrelated sequence of similar length (150 amino acids) derived from the SLN1 gene. pSte2_–_Sho1-D contains a truncation of Sho1 in which the SH3 domain is replaced by the PBS2 coding region. (B) Sho1_–_Ste2 chimeric constructs were transformed into the yeast strain MY007 (ssk2Δ ssk22Δ sho1Δ), and the osmosensitivity of the transformed cells was tested on YPD plates containing 1.5 M sorbitol. (C) Tyrosine phosphorylation of Hog1 at various times after the addition of 0.4 M NaCl to the medium. The same cells used in (A) were subjected to immunoblot analysis using anti-phosphotyrosine antibody 4G10.
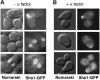
Fig. 5. Localization of Sho1 to sites of polarized growth. Localization of the Sho1 protein was determined using a Sho1_–_GPF fusion construct (pSHO1_–_GFP). Expression of the Sho1_–_GFP fusion protein is maintained at physiological levels by using a low copy-number vector (pRS416) and the promoter of the SHO1 gene itself. (A) The majority of the Sho1_–_GFP protein is localized to the incipient bud (top panel), and to the plasma membrane of the growing bud (lower panels), during vegetative growth. pSHO1_–_GFP is expressed in the yeast strain FP66 (sho1Δ), and exponentially growing cells in YPD medium were examined by fluorescence microscopy. (B) Sho1_–_GFP is localized at the shmoo tip during the mating response. pSHO1_–_GFP is expressed in the wild-type strain TM141 in the presence of 3 µM α mating factor.
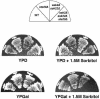
Fig. 6. Deletion of the STE20 gene in cells which are defective for the SLN1 branch of the HOG pathway (in this case, ssk2Δ ssk22Δ double mutant) leads to osmosensitivity with galactose but not dextrose, as the sole carbon source. Wild-type strain TM141 (WT), and mutant strains FP58 (ste20Δ), TM252 (ssk2Δ ssk22Δ) and DR76 (ssk2Δ ssk22Δ ste20Δ) were streaked onto rich media containing 2% glucose (YPD) or 2% galactose (YPGal) with or without 1.5 M sorbitol.
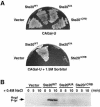
Fig. 7. Overexpression of Cdc42 or the PAK homolog Cla4 rescues the osmosensitive phenotype caused by a ste20Δ mutation. Strain DR76 (ssk2Δ ssk22Δ ste20Δ) was transformed with the galactose promoter containing pYES2 (Vector), wild-type CDC42 inserted into pYES2 (Cdc42) or a high copy-number plasmid, pCC1079 (Chen et al., 1997) containing the wild-type CLA4 gene under its own promoter (Cla4). Transformants were streaked onto YPGal or on YPGal containing 1.5 M sorbitol and incubated at 30°C.
Fig. 8. Both the kinase activity and the CRIB domain of Ste20 are required for the osmotic stress response mediated by the SHO1 branch of the HOG pathway. (A) Strain DR76 (ssk2Δ ssk22Δ ste20Δ) was transformed with a multicopy (2 µm) plasmid containing either wild-type STE20, the kinase inactive _STE20_K/A allele or the _STE20Δ_CRB mutant allele lacking the Cdc42-binding domain. Transformants were streaked onto selective media containing galactose as the sole carbon source in the absence (upper panel) or presence (lower panel) of 1.5 M sorbitol. Whereas wild-type STE20 could rescue the osmosensitivity of the ste20Δ strain DR76, both mutant alleles of STE20 and the high copy-number expression vector alone failed to suppress the osmosensitive phenotype. (B) Activation of the Hog1 MAP kinase by osmotic stress in the transformants detailed in (A) was determined by immunoblot analysis using the 4G10 anti-phosphotyrosine antibody. Exponentially growing cultures were harvested before (0), or at the indicated times after, the addition of 0.4 M NaCl, and subjected to immunoblot analysis.
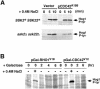
Fig. 9. Effect of dominant-active and dominant-negative CDC42 on Hog1 activation. (A) The dominant-negative _CDC42_A188 allele inhibits the SHO1 branch of the HOG pathway. Wild-type strain TM141 (SSK2+ SSK22+) and a mutant strain TM252 (ssk2Δ ssk22Δ) were transformed with an expression plasmid pYES2 with a galactose-inducible promoter (Vector), or pYES2 containing the dominant-negative _CDC42_A188 allele (pCDC42A188). Expression of the _CDC42_A188 allele was induced by the addition of 2% galactose to exponentially growing cultures containing non-repressing raffinose as carbon source. Two-and-a-half hours after the addition of galactose, the ability of the cells to activate the HOG pathway was determined by monitoring Hog1 tyrosine phosphorylation following osmotic shock. Samples were taken before (time 0), and at 5 and 10 min after the addition of 0.4 M NaCl for 4G10 immunoblot analysis. Expression of the dominant-negative _CDC42_A188 allele significantly inhibited signaling through the SHO1 branch of the HOG pathway in ssk2Δ ssk22Δ cells. In contrast, activation of Hog1 was unaffected in wild-type cells (SSK2+ SSK22+), in which the SLN1 branch is intact. (B) Expression of the dominant-active _CDC42_V12 allele activates the HOG pathway in the absence of osmotic stress. Strain TM252 (ssk2Δ ssk22Δ) was transformed with galactose-inducible constructs of dominant-active _CDC42_V12 or dominant-active _RHO1_V19. Hog1 activation was assessed as above by 4G10 immunoblot analysis. Exponentially growing cells in non-inducing raffinose media (time 0) were either untreated (–) or treated with NaCl for 5 min (+) as controls for baseline and maximal Hog1 activation, respectively. At the indicated times after addition of 2% galactose to induce expression of _RHO1_V19 or _CDC42_V12, samples were harvested (without osmotic shock) for 4G10 analysis. At 6 h of _CDC42_V12 expression, weak, but significant, phosphorylation of the Hog1 kinase was detected in the absence of osmotic stress.
Similar articles
- Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway.
Tatebayashi K, Yamamoto K, Tanaka K, Tomida T, Maruoka T, Kasukawa E, Saito H. Tatebayashi K, et al. EMBO J. 2006 Jul 12;25(13):3033-44. doi: 10.1038/sj.emboj.7601192. Epub 2006 Jun 15. EMBO J. 2006. PMID: 16778768 Free PMC article. - A docking site determining specificity of Pbs2 MAPKK for Ssk2/Ssk22 MAPKKKs in the yeast HOG pathway.
Tatebayashi K, Takekawa M, Saito H. Tatebayashi K, et al. EMBO J. 2003 Jul 15;22(14):3624-34. doi: 10.1093/emboj/cdg353. EMBO J. 2003. PMID: 12853477 Free PMC article. - Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42.
Reiser V, Salah SM, Ammerer G. Reiser V, et al. Nat Cell Biol. 2000 Sep;2(9):620-7. doi: 10.1038/35023568. Nat Cell Biol. 2000. PMID: 10980703 - [Mechanism of HOG-MAPK pathway in regulating mycotoxins formation under environmental stresses].
Ma Y, Li M, Wang Z, Liao L, Zheng Y, Liu Y. Ma Y, et al. Sheng Wu Gong Cheng Xue Bao. 2022 Jul 25;38(7):2433-2446. doi: 10.13345/j.cjb.220060. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 35871615 Review. Chinese. - Regulation of the osmoregulatory HOG MAPK cascade in yeast.
Saito H, Tatebayashi K. Saito H, et al. J Biochem. 2004 Sep;136(3):267-72. doi: 10.1093/jb/mvh135. J Biochem. 2004. PMID: 15598881 Review.
Cited by
- Phosphoproteome dynamics of Saccharomyces cerevisiae under heat shock and cold stress.
Kanshin E, Kubiniok P, Thattikota Y, D'Amours D, Thibault P. Kanshin E, et al. Mol Syst Biol. 2015 Jun 3;11(6):813. doi: 10.15252/msb.20156170. Mol Syst Biol. 2015. PMID: 26040289 Free PMC article. - A framework for mapping, visualisation and automatic model creation of signal-transduction networks.
Tiger CF, Krause F, Cedersund G, Palmér R, Klipp E, Hohmann S, Kitano H, Krantz M. Tiger CF, et al. Mol Syst Biol. 2012 Apr 24;8:578. doi: 10.1038/msb.2012.12. Mol Syst Biol. 2012. PMID: 22531118 Free PMC article. - A biochemical genomics screen for substrates of Ste20p kinase enables the in silico prediction of novel substrates.
Annan RB, Lee AY, Reid ID, Sayad A, Whiteway M, Hallett M, Thomas DY. Annan RB, et al. PLoS One. 2009 Dec 16;4(12):e8279. doi: 10.1371/journal.pone.0008279. PLoS One. 2009. PMID: 20020052 Free PMC article. - Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway.
Tatebayashi K, Tanaka K, Yang HY, Yamamoto K, Matsushita Y, Tomida T, Imai M, Saito H. Tatebayashi K, et al. EMBO J. 2007 Aug 8;26(15):3521-33. doi: 10.1038/sj.emboj.7601796. Epub 2007 Jul 12. EMBO J. 2007. PMID: 17627274 Free PMC article. - Two adjacent docking sites in the yeast Hog1 mitogen-activated protein (MAP) kinase differentially interact with the Pbs2 MAP kinase kinase and the Ptp2 protein tyrosine phosphatase.
Murakami Y, Tatebayashi K, Saito H. Murakami Y, et al. Mol Cell Biol. 2008 Apr;28(7):2481-94. doi: 10.1128/MCB.01817-07. Epub 2008 Jan 22. Mol Cell Biol. 2008. PMID: 18212044 Free PMC article.
References
- Bagrodia S., Dérijard,B., Davis,R.J. and Cerione,R.A. (1995) Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem., 270, 27995–27998. - PubMed
- Cvrcková F., De Virgilio,C., Manser,E., Pringle,J.R. and Nasmyth,K. (1995) Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev., 9, 1817–1830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous