Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing - PubMed (original) (raw)
Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing
J Y Lee et al. J Cell Biol. 2000.
Abstract
Several recent studies suggest the isolation of stem cells in skeletal muscle, but the functional properties of these muscle-derived stem cells is still unclear. In the present study, we report the purification of muscle-derived stem cells from the mdx mouse, an animal model for Duchenne muscular dystrophy. We show that enrichment of desmin(+) cells using the preplate technique from mouse primary muscle cell culture also enriches a cell population expressing CD34 and Bcl-2. The CD34(+) cells and Bcl-2(+) cells were found to reside within the basal lamina, where satellite cells are normally found. Clonal isolation and characterization from this CD34(+)Bcl-2(+) enriched population yielded a putative muscle-derived stem cell, mc13, that is capable of differentiating into both myogenic and osteogenic lineage in vitro and in vivo. The mc13 cells are c-kit and CD45 negative and express: desmin, c-met and MNF, three markers expressed in early myogenic progenitors; Flk-1, a mouse homologue of KDR recently identified in humans as a key marker in hematopoietic cells with stem cell-like characteristics; and Sca-1, a marker for both skeletal muscle and hematopoietic stem cells. Intramuscular, and more importantly, intravenous injection of mc13 cells result in muscle regeneration and partial restoration of dystrophin in mdx mice. Transplantation of mc13 cells engineered to secrete osteogenic protein differentiate in osteogenic lineage and accelerate healing of a skull defect in SCID mice. Taken together, these results suggest the isolation of a population of muscle-derived stem cells capable of improving both muscle regeneration and bone healing.
Figures
Figure 1
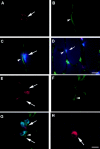
Colocalization of CD34 and Bcl-2 positive cells with laminin/collagen type IV. Muscle sections from normal mice were stained with anti-CD34 antibody and visualized with fluorescence (A, arrow). The sections were costained with antilaminin antibody to outline the basal lamina (B, arrowhead), and Hoechst to demonstrate nuclei (C and D, blue fluorescence). The CD34 positive cells were located within the basal lamina (C and D). The muscle sections were also stained with Bcl-2, collagen type IV, and Hoechst in a similar manner. The Bcl-2 positive cells (E, arrow), which colocalized with nuclei staining (G), were found located within the basal lamina (F and G). M-cadherin staining showed that satellite cells are found beneath the basal lamina at similar locations as CD34+ or Bcl-2+ cells (H). Bar: (A–C, E–H) 10 μm; (D) 25 μm.
Figure 2
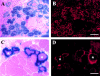
Morphologic change and expression of osteocalcin by mc13 cells with exposure to rhBMP-2. Mc13 cells were incubated in growth media without rhBMP-2 for 6 d. When cells became >50% confluent, they began to fuse and form multinucleated myotubes (A). When mc13 cells were incubated in growth media containing 200 ng/ml rhBMP-2, cells remained mononucleated and did not fuse (B). When cells reached >90% confluency without rhBMP-2, almost all the cells fused to form myotubes (C). With rhBMP-2, when cells reached >90% confluency, round, hypertrophic cells began to appear in the culture (D, arrows). These round, hypertrophic cells were highly positive for osteocalcin expression (E, arrows). Bar, 50 μm.
Figure 3
The effect of rhBMP-2 on the expression of desmin and alkaline phosphatase by the mc13 cells. Desmin staining of freshly isolated mc13 clones (A) showed that all of these cells express desmin. The phase-contrast view (B) is shown to demonstrate that a high percentage of the cells were desmin positive. Mc13 cells were incubated in growth media containing 200 ng/ml rhBMP-2 for 6 d. Cells were then stained for desmin expression, and percent desmin positive cells calculated by visualization with immunofluorescence. As a control, mc13 cells were grown in parallel without addition of rhBMP-2. When grown without rhBMP-2, mc13 cells remained uniformly (90–100%) desmin positive (C). With exposure to rhBMP-2, there is a significant decrease (*P < 0.05) in relative number of desmin positive cells (30–40%) within 6 d (C). The npmc and mc13 cells were also analyzed for expression of alkaline phosphatase after 6 d of growth with and without exposure to 200 ng/ml rhBMP-2. With rhBMP-2 stimulation, mc13 cells show a >600-fold increase in alkaline phosphatase activity than nonstimulated cells (D). The npmc show only minimal alkaline phosphatase activity with or without rhBMP-2 (D). *, Indicates a significant difference using the t test (P < 0.05). Bar, 100 μm.
Figure 3
The effect of rhBMP-2 on the expression of desmin and alkaline phosphatase by the mc13 cells. Desmin staining of freshly isolated mc13 clones (A) showed that all of these cells express desmin. The phase-contrast view (B) is shown to demonstrate that a high percentage of the cells were desmin positive. Mc13 cells were incubated in growth media containing 200 ng/ml rhBMP-2 for 6 d. Cells were then stained for desmin expression, and percent desmin positive cells calculated by visualization with immunofluorescence. As a control, mc13 cells were grown in parallel without addition of rhBMP-2. When grown without rhBMP-2, mc13 cells remained uniformly (90–100%) desmin positive (C). With exposure to rhBMP-2, there is a significant decrease (*P < 0.05) in relative number of desmin positive cells (30–40%) within 6 d (C). The npmc and mc13 cells were also analyzed for expression of alkaline phosphatase after 6 d of growth with and without exposure to 200 ng/ml rhBMP-2. With rhBMP-2 stimulation, mc13 cells show a >600-fold increase in alkaline phosphatase activity than nonstimulated cells (D). The npmc show only minimal alkaline phosphatase activity with or without rhBMP-2 (D). *, Indicates a significant difference using the t test (P < 0.05). Bar, 100 μm.
Figure 3
The effect of rhBMP-2 on the expression of desmin and alkaline phosphatase by the mc13 cells. Desmin staining of freshly isolated mc13 clones (A) showed that all of these cells express desmin. The phase-contrast view (B) is shown to demonstrate that a high percentage of the cells were desmin positive. Mc13 cells were incubated in growth media containing 200 ng/ml rhBMP-2 for 6 d. Cells were then stained for desmin expression, and percent desmin positive cells calculated by visualization with immunofluorescence. As a control, mc13 cells were grown in parallel without addition of rhBMP-2. When grown without rhBMP-2, mc13 cells remained uniformly (90–100%) desmin positive (C). With exposure to rhBMP-2, there is a significant decrease (*P < 0.05) in relative number of desmin positive cells (30–40%) within 6 d (C). The npmc and mc13 cells were also analyzed for expression of alkaline phosphatase after 6 d of growth with and without exposure to 200 ng/ml rhBMP-2. With rhBMP-2 stimulation, mc13 cells show a >600-fold increase in alkaline phosphatase activity than nonstimulated cells (D). The npmc show only minimal alkaline phosphatase activity with or without rhBMP-2 (D). *, Indicates a significant difference using the t test (P < 0.05). Bar, 100 μm.
Figure 4
In vivo differentiation of mc13 cells into myogenic lineage after i.m. and i.v. injection. The mc13 cells were stably transfected with a plasmid DNA construct encoding LacZ, dystrophin, and neomycin resistance genes and injected intramuscularly into hind limbs of mdx mice. After 7 d, mice were killed and hind limb musculature was isolated for histology. Many LacZ positive myofibers (A) were found at the injected site that colocalized with dystrophin positive myofibers (B). Some LacZ (C,*) and dystrophin positive myofibers (D,*) were also found in the hind limb muscle of mdx mice after i.v. injection of mc13. The number of myofiber that coexpressed LacZ and dystrophin was counted (E) and compared between the i.m. (IM) and i.v. (IV) groups. Bar: (A and B) 100 μm; (C and D) 50 μm.
Figure 4
In vivo differentiation of mc13 cells into myogenic lineage after i.m. and i.v. injection. The mc13 cells were stably transfected with a plasmid DNA construct encoding LacZ, dystrophin, and neomycin resistance genes and injected intramuscularly into hind limbs of mdx mice. After 7 d, mice were killed and hind limb musculature was isolated for histology. Many LacZ positive myofibers (A) were found at the injected site that colocalized with dystrophin positive myofibers (B). Some LacZ (C,*) and dystrophin positive myofibers (D,*) were also found in the hind limb muscle of mdx mice after i.v. injection of mc13. The number of myofiber that coexpressed LacZ and dystrophin was counted (E) and compared between the i.m. (IM) and i.v. (IV) groups. Bar: (A and B) 100 μm; (C and D) 50 μm.
Figure 6
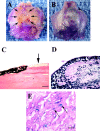
The inability of BMP-2 expressing npmc to differentiate into osteogenic lineage. The npmc were isolated from a male mdx mouse and injected in the hind limb muscle of a female SCID mice. We have observed that npmc genetically engineered to express BMP-2 also leads to ectopic bone formation when injected in skeletal muscle. A, The FISH technique was used to identify the Y chromosome positive cells (donor cells) and revealed that the injected cells were located outside of the osteoid (B, arrow); a complete absence of Y chromosome positive cells was found within the newly formed osteoid (C, arrows). These cells were therefore incapable of differentiating into osteogenic lineage in vivo. Bar: (A) 100 μm; (B and C) 50 μm.
Figure 7
Enhancement of bone healing by rhBMP-2 producing mc13 cells. A 5-mm skull defect was created in a SCID mice using a dental burr, and the defect was filled with a collagen sponge seeded with mc13, with or without adBMP-2 transduction. The mice were killed at 14 d and analyzed grossly and microscopically for healing of the defect. A gross specimen from the control group was treated with collagen sponge seeded with mc13 without adBMP-2. At 14 d, there was no apparent healing of the skull defect (A, arrows). A representative specimen from mice treated with collagen sponge seeded with mc13 transduced with adBMP-2 (B). There was almost a complete closure of the defect within 14 d (B). A von Kossa staining of the histological specimen from the control group showed no evidence of new bone formation (C, arrow shows defect site). von Kossa stain of the adBMP-2–treated group showed robust bone formation at 14 d (D). The mc13 cells transduced with adBMP-2 were followed using the LacZ staining (E). The vast majority of the β-galactosidase expressing nuclei (>95%) was found within the newly formed bone (E, arrows). Bar: (C and D) 100 μm; (E) 50 μm.
Figure 5
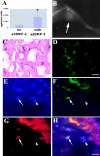
In vivo differentiation of mc13 cells into osteogenic lineage after genetic engineering to express BMP-2. The amount of BMP-2 secreted by the mc13 cells that were transduced with adBMP-2 was found significantly higher (*P < 0.05) than the nontransduced mc13 cells (A). 0.5–1.0 × 106 cells genetically engineered to express BMP-2 were injected into hind limbs of SCID mice. After 14 d, mice were killed, and the hind limb muscle tissues were analyzed radiographically for evidence of bone formation. There was a robust ectopic bone formation (seen radiographically) within skeletal muscle in all mice injected with mc13 cells transduced with adBMP-2 (B, arrow). The injected muscle containing the ectopic bone was then harvested and stained for β-galactosidase activity to locate injected cells. The LacZ positive cells were uniformly found within the lacunae, a location where osteoblasts and osteocytes are normally found (C). The ectopic bone was also stained for presence of dystrophin. As indicated by green fluorescence, the ectopic bone contained abundant cells expressing dystrophin, confirming that mc13 cells were active participants in formation of bone (D). To determine whether the genetically engineered mc13 expressing BMP-2 can express bone protein, we colocalized β-galactosidase expressing nuclei, osteocalcin expression, and nuclei staining (DAPI) by immunohistochemistry (E–H). We identified nuclei expressing β-galactosidase (see Fig. 6 F, arrow, FITC/green) that expressed osteocalcin (see Fig. 6 G, arrow, cy3/red) and colocalized with nuclei staining (see Fig. 6 E, arrow, DAPI/blue). The triple colocalization of DAPI/osteocalcin and β-galactosidase (Fig. 6 H, arrows) suggests that the genetically engineered mc13 can express bone protein (osteocalcin). We have also observed β-galactosidase expressing nuclei (Fig. 6 F, arrowhead) that were not colocalized with osteocalcin expressing cells (Fig. 6 G, arrowhead), suggesting that some of the engineered mc13 were not expressing osteocalcin (Fig. 6, E–H, arrowheads). Bar: (C and D) 50 μm; (E–H) 25 μm.
Similar articles
- Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells.
Jankowski RJ, Haluszczak C, Trucco M, Huard J. Jankowski RJ, et al. Hum Gene Ther. 2001 Apr 10;12(6):619-28. doi: 10.1089/104303401300057306. Hum Gene Ther. 2001. PMID: 11426462 - Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice.
Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, Fortunato F, El Fahime M, D'Angelo MG, Caron NJ, Constantin G, Paulin D, Scarlato G, Bresolin N. Torrente Y, et al. J Cell Biol. 2001 Jan 22;152(2):335-48. doi: 10.1083/jcb.152.2.335. J Cell Biol. 2001. PMID: 11266450 Free PMC article. - Human muscle-derived cell populations isolated by differential adhesion rates: phenotype and contribution to skeletal muscle regeneration in Mdx/SCID mice.
Chirieleison SM, Feduska JM, Schugar RC, Askew Y, Deasy BM. Chirieleison SM, et al. Tissue Eng Part A. 2012 Feb;18(3-4):232-41. doi: 10.1089/ten.TEA.2010.0553. Epub 2011 Oct 11. Tissue Eng Part A. 2012. PMID: 21854253 Free PMC article. - A new look at the origin, function, and "stem-cell" status of muscle satellite cells.
Seale P, Rudnicki MA. Seale P, et al. Dev Biol. 2000 Feb 15;218(2):115-24. doi: 10.1006/dbio.1999.9565. Dev Biol. 2000. PMID: 10656756 Review. - Stem cells in adult skeletal muscle.
Asakura A. Asakura A. Trends Cardiovasc Med. 2003 Apr;13(3):123-8. doi: 10.1016/s1050-1738(03)00024-0. Trends Cardiovasc Med. 2003. PMID: 12691677 Review.
Cited by
- Skeletal Muscle Regenerative Engineering.
Tang X, Daneshmandi L, Awale G, Nair LS, Laurencin CT. Tang X, et al. Regen Eng Transl Med. 2019 Sep;5(3):233-251. doi: 10.1007/s40883-019-00102-9. Epub 2019 Apr 2. Regen Eng Transl Med. 2019. PMID: 33778155 Free PMC article. - Research progress in muscle-derived stem cells: Literature retrieval results based on international database.
Zhang L, Wang W. Zhang L, et al. Neural Regen Res. 2012 Apr 5;7(10):784-91. doi: 10.3969/j.issn.1673-5374.2012.10.010. Neural Regen Res. 2012. PMID: 25737703 Free PMC article. - Mesenchymal progenitor cells derived from traumatized muscle enhance neurite growth.
Jackson WM, Alexander PG, Bulken-Hoover JD, Vogler JA, Ji Y, McKay P, Nesti LJ, Tuan RS. Jackson WM, et al. J Tissue Eng Regen Med. 2013 Jun;7(6):443-51. doi: 10.1002/term.539. Epub 2012 May 3. J Tissue Eng Regen Med. 2013. PMID: 22552971 Free PMC article. - Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells.
Jackson WM, Nesti LJ, Tuan RS. Jackson WM, et al. Expert Opin Biol Ther. 2010 Apr;10(4):505-17. doi: 10.1517/14712591003610606. Expert Opin Biol Ther. 2010. PMID: 20218920 Free PMC article. Review. - Skeletal muscle stem cells.
Chen JC, Goldhamer DJ. Chen JC, et al. Reprod Biol Endocrinol. 2003 Nov 13;1:101. doi: 10.1186/1477-7827-1-101. Reprod Biol Endocrinol. 2003. PMID: 14614776 Free PMC article. Review.
References
- Andrews R.G., Singer J.W., Bernstein I.D. Monoclonal antibody 12-8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood. 1986;67:842–845. - PubMed
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Egicjo C., Ishihara T., Nonak I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1989;333:861–863. - PubMed
- Barch, M.J. 1991. Chromosome analysis. In The ACT Cytogenetics Laboratory Manual. 2nd edition. Raven Press, Ltd., NY, NY. 349.
- Baroffio A., Hamann M., Bernheim L., Bochaton-Piallat M.L., Gabbiani G., Bader C.R. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. - PubMed
- Beauchamps J.R., Morgan J.E., Pagel C.N., Partridge T.A. Quantitative studies of the efficacy of myoblast transplantation. Muscle Nerve. 1994;18:S261.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous