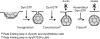Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis - PubMed (original) (raw)
Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis
S Sever et al. J Cell Biol. 2000.
Abstract
The GTPase dynamin is essential for receptor-mediated endocytosis, but its function remains controversial. A domain of dynamin, termed the GTPase effector domain (GED), controls dynamin's high stimulated rates of GTP hydrolysis by functioning as an assembly-dependent GAP. Dyn(K694A) and dyn(R725A) carry point mutations within GED resulting in reduced assembly stimulated GTPase activity. Biotinylated transferrin is more rapidly sequestered from avidin in cells transiently overexpressing either of these two activating mutants (Sever, S., A.B. Muhlberg, and S.L. Schmid. 1999. Nature. 398:481-486), suggesting that early events in receptor-mediated endocytosis are accelerated. Using stage-specific assays and morphological analyses of stably transformed cells, we have identified which events in clathrin-coated vesicle formation are accelerated by the overexpression of dyn(K694A) and dyn(R725A). Both mutants accelerate the formation of constricted coated pits, which we identify as the rate limiting step in endocytosis. Surprisingly, overexpression of dyn(R725A), whose primary defect is in stimulated GTP hydrolysis, but not dyn(K694A), whose primary defect is in self-assembly, inhibited membrane fission leading to coated vesicle release. Together, our data support a model in which dynamin functions like a classical GTPase as a key regulator of clathrin-mediated endocytosis.
Figures
Figure 1
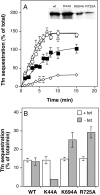
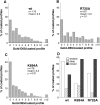
Transferrin internalization is increased in cells stably expressing the dyn (K694A) and dyn(R725A). (A) Cells expressing either wild-type dynamin (▪), dyn (K44A) (⋄), dyn(K694A) (○), or dyn(R725A) (▵) were induced for 48 h by removal of tetracycline. The inset indicates the expression levels for exogenous dynamin from a single representative assay as analyzed by Western blotting. Internalization of transferrin (Tfn) was measured by incubating cells with 4 μg/ml B-Tfn for the indicated times at 37°C before measuring intracellular Tfn based on its inaccessibility to avidin (see Materials and Methods). (B) The initial rates of Tfn internalization, which were determined within the first 5 min of uptake for the induced cells, are shown (solid bars) relative to noninduced control cells (open bars). Results in both panels are the average ± SD of five independent experiments.
Figure 2
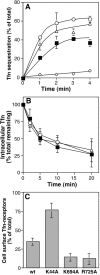
Effects of dynamin:GTP on the transferrin receptor cycle. Stably transformed tTA-HeLa cells expressing dyn(wt) (▪), dyn (K44A) (⋄), dyn(K694A) (○), or dyn(R725A) (▵) were induced for ∼48 h and analyzed in different assays. (A) Single-round kinetics of Tfn internalization were determined by first incubating cells with an excess of BSS-Tfn for 30 min on ice. After the unbound ligand was removed, internalization of prebound BSS-Tfn was determined at early time points by incubating cells at 37°C. (B) Recycling of Tfn receptors from endosomes was assessed by first loading cells with BSS-Tfn at 37°C to steady state. Surface-bound BSS-Tfn was masked by avidin at 4°C, and the recycling of intracellular BSS-Tfn was determined after incubation at 37°C for indicated times (see Materials and Methods). (C) Effects of dynamin mutants on the cellular distribution of the Tfn receptor was determined by allowing cells to bind BSS-Tfn on ice or at 37°C for 2 h. Unbound ligand was subsequently removed by extensive washing, and cell-associated BSS-Tfn was determined using the ELISA assay. The proportion of Tfn receptors on the surface (average ± SD, n = 3) was calculated as the ratio between the BSS-Tfn bound at 0 versus 37°C (van der Sluijs et al. 1992).
Figure 3
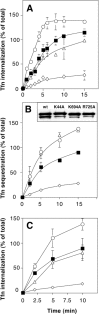
Membrane fission is inhibited in cells overexpressing dyn(R725A). (A) Stably transformed tTA-HeLa cells expressing dyn (wt) (▪), dyn(K44A) (⋄), dyn(K694A) (○), or dyn(R725A) (▵) were induced for 48 h and internalization of Tfn into the sealed coated vesicles was assessed by MesNa resistance (see Materials and Methods). Results shown are the average ± SD of five independent experiments. (B and C) BHK cells were cotransfected with cDNAs encoding the human transferrin (Tfn) receptor and either wild-type dynamin (▪), dyn(K44A) (⋄), dyn(K694A) (○), or dyn(R725A) (▵) and cultured for an additional 24 h before harvesting for endocytosis assays as described in Fig. 1. Inset in B shows Western blot analysis of the same number of BHK cells transiently transfected with cDNAs encoding different dynamin mutants. The lower band is a degradation product of dynamin seen at high levels of overexpression. The extent of BSS-Tfn internalization was assessed either by avidin accessibility (B) or MesNa resistance (C, see Materials and Methods). Shown are the average ± SD of three independent experiments with at least 60% transfection efficiency.
Figure 4
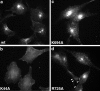
Effect of dynamin mutants on rhodamine-Tfn internalization. Stably transformed tTA-HeLa cells expressing dyn(wt) (a), dyn(K44A) (b), dyn(K694A) (c), or dyn(R725A) (d) were grown on coverslips for ∼48 h, during which dynamin expression was induced by the removal of tet. Cells were incubated with rhodamine-Tfn for 5 min at 37°C before fixation and analysis by fluorescence microscopy.
Figure 6
Quantitative analysis of TfnR sorting and endocytic intermediates in cells expressing wt and mutant dynamins. A–C show the distribution of coated profiles containing D65-gold for cells expressing dyn(wt) (A), dyn(K694A) (B), and dyn(R725A) (C). Cells overexpressing dyn(K694A) mutant show a statistically different distribution of gold particles relative to wt cells as assessed by the analysis of variance statistics (ANOVA). D shows the distribution of coated endocytic structures in wt and mutant cells. Quantitation was performed at the microscope as described in Materials and Methods. The total number of coated pits scored for this analysis was 68, 86, and 89 for wt, dyn(K694A) and dyn(R725A), respectively.
Figure 5
EM analysis of coated profiles in cells expressing wt and mutant dynamins. Transformed tTA-HeLa cells, which were induced to express either dyn(wt) (A and D), dyn (K694A) (B and E), or dyn(R725A) (C and F), were grown on coverslips. Cells were incubated with gold-conjugated monoclonal anti–human TfnR antibody (D65-gold) for 30 min at 37°C before fixation, staining, and embedding in Epon for thin section analysis. Open arrowhead (A) points to a shallow coated pit; closed arrowheads in (A–C) point to closed pits that are most likely open to the cell surface in subsequent sections (D–F); and arrows point to deep coated pits where openings are visible. E indicates endosomal structures containing D65-gold. Bar, 200 nm.
Figure 7
Kinetic model for the role of dynamin:GTP in endocytosis. Shown are the rate limiting steps in coated vesicle formation measured in cells overexpressing wt and mutant dynamins as detected biochemically. Our results suggest that the rate limiting step controlled by dynamin:GTP is the formation of the constricted pit needed to prime emerging vesicles for fission. Once the pit becomes constricted (or primed), it is rapidly consumed in the fission step to release a coated vesicle. Overexpression of dyn(K694A), which is impaired in self-assembly, increased the rate of formation of constricted coated pits and, correspondingly, the overall rate of endocytic-coated vesicle formation. In contrast, overexpression of the dyn(R725A), which is defective in GTP hydrolysis–triggered disassembly, increased the rate of formation of constricted coated pits without increasing the rate of coated vesicle release, so that membrane fission became rate-limiting. This suggests that assembled, GTPase–defective dynamin inhibits membrane fission.
Similar articles
- Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages.
Damke H, Binns DD, Ueda H, Schmid SL, Baba T. Damke H, et al. Mol Biol Cell. 2001 Sep;12(9):2578-89. doi: 10.1091/mbc.12.9.2578. Mol Biol Cell. 2001. PMID: 11553700 Free PMC article. - Role of dynamin in clathrin-coated vesicle formation.
Baba T, Damke H, Hinshaw JE, Ikeda K, Schmid SL, Warnock DE. Baba T, et al. Cold Spring Harb Symp Quant Biol. 1995;60:235-42. doi: 10.1101/sqb.1995.060.01.027. Cold Spring Harb Symp Quant Biol. 1995. PMID: 8824396 No abstract available. - Induction of mutant dynamin specifically blocks endocytic coated vesicle formation.
Damke H, Baba T, Warnock DE, Schmid SL. Damke H, et al. J Cell Biol. 1994 Nov;127(4):915-34. doi: 10.1083/jcb.127.4.915. J Cell Biol. 1994. PMID: 7962076 Free PMC article. - Dissecting dynamin's role in clathrin-mediated endocytosis.
Mettlen M, Pucadyil T, Ramachandran R, Schmid SL. Mettlen M, et al. Biochem Soc Trans. 2009 Oct;37(Pt 5):1022-6. doi: 10.1042/BST0371022. Biochem Soc Trans. 2009. PMID: 19754444 Free PMC article. Review. - Dynamin GTPase, a force-generating molecular switch.
Warnock DE, Schmid SL. Warnock DE, et al. Bioessays. 1996 Nov;18(11):885-93. doi: 10.1002/bies.950181107. Bioessays. 1996. PMID: 8939066 Review.
Cited by
- Mitochondrial dynamics and division in budding yeast.
Shaw JM, Nunnari J. Shaw JM, et al. Trends Cell Biol. 2002 Apr;12(4):178-84. doi: 10.1016/s0962-8924(01)02246-2. Trends Cell Biol. 2002. PMID: 11978537 Free PMC article. Review. - The Toca-1-N-WASP complex links filopodial formation to endocytosis.
Bu W, Chou AM, Lim KB, Sudhaharan T, Ahmed S. Bu W, et al. J Biol Chem. 2009 Apr 24;284(17):11622-36. doi: 10.1074/jbc.M805940200. Epub 2009 Feb 11. J Biol Chem. 2009. PMID: 19213734 Free PMC article. - Dynamin assembly strategies and adaptor proteins in mitochondrial fission.
Bui HT, Shaw JM. Bui HT, et al. Curr Biol. 2013 Oct 7;23(19):R891-9. doi: 10.1016/j.cub.2013.08.040. Curr Biol. 2013. PMID: 24112988 Free PMC article. Review. - The glomerular filtration barrier: a structural target for novel kidney therapies.
Daehn IS, Duffield JS. Daehn IS, et al. Nat Rev Drug Discov. 2021 Oct;20(10):770-788. doi: 10.1038/s41573-021-00242-0. Epub 2021 Jul 14. Nat Rev Drug Discov. 2021. PMID: 34262140 Free PMC article. Review. - Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis.
Kirchhausen T, Macia E, Pelish HE. Kirchhausen T, et al. Methods Enzymol. 2008;438:77-93. doi: 10.1016/S0076-6879(07)38006-3. Methods Enzymol. 2008. PMID: 18413242 Free PMC article.
References
- Baba T., Ueda H., Terada N., Fujii Y., Ohno S. Immunocytochemical study of endocytotic structures accumulated in HeLa cells transformed with a temperature-sensitive mutant of dynamin. J. Histochem. Cytochem. 1999;47:637–648. - PubMed
- Carr J.F., Hinshaw J.E. Dynamin assembles into spirals under physiological salt conditions upon the addition of GDP and gamma-phosphate analogues. J. Biol. Chem. 1997;272:28030–28035. - PubMed
- Ciechanover A., Schwartz A.L., Dautry-Varsat A., Lodish H.F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J. Biol. Chem. 1983;258:9681–9689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous